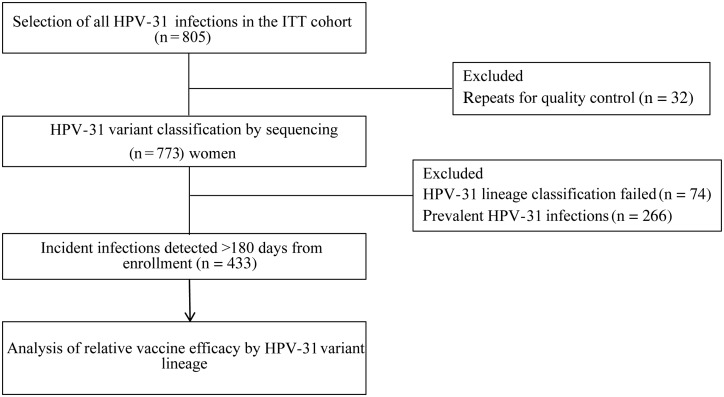
Figure 2.
Expanded study design to assess the relative efficacy of the bivalent human papillomavirus (HPV) vaccine against HPV-31 variant infections. Samples in which HPV-31 was previously detected [6] were selected for variant lineage classification, and incident infections were evaluated for lineage-specific vaccine efficacy. A total of 433 incident HPV-31 infections, classified into variant lineages, were evaluated for relative vaccine efficacy, based on infection duration ([1] transient infection or [2] persistent infection and/or cervical intraepithelial neoplasia grade 2/3). Prevalent infections (n = 266), defined as HPV-31 detected within 180 days of study enrollment, were excluded in this analysis. Repeats for QC were an additional sample selected from a different time point in 32 women. Abbreviation: ITT, intention to treat.