Figure 2.
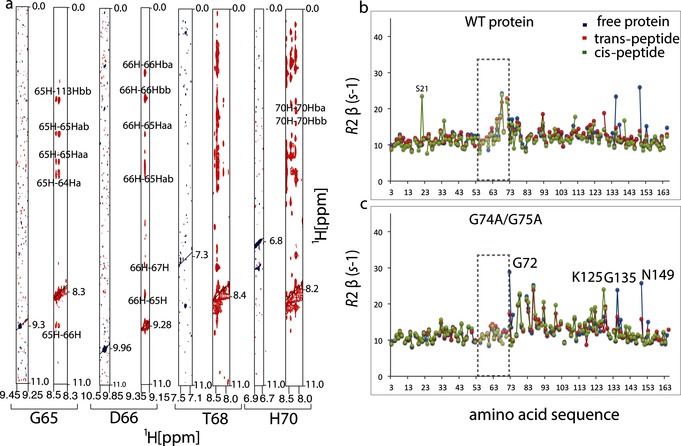
Loop residues are less mobile in the glycine double mutant. a) Cross‐sections of [1H,1H]‐NOESY spectra showing cross peaks for G65, D66, T68, and H70 for WT cyclophilin (blue) and G74A and G75A mutant (red). Increased intensities of cross peaks indicate that loop residues make substantial contact with the rest of the protein and that they are less mobile in the mutant than in the WT protein. b), c) Transverse R 2 NMR relaxation rates plotted as functions of the amino acid residues for the free WT cyclophilin and mutant (blue), and bound to the trans‐peptide (red) and cis‐peptide (green). In the double glycine mutant, the increased R 2 rates of loop residues reduce to R 2 values typical of the rest of the protein (gray rectangle), except around the residues near the mutation site.
