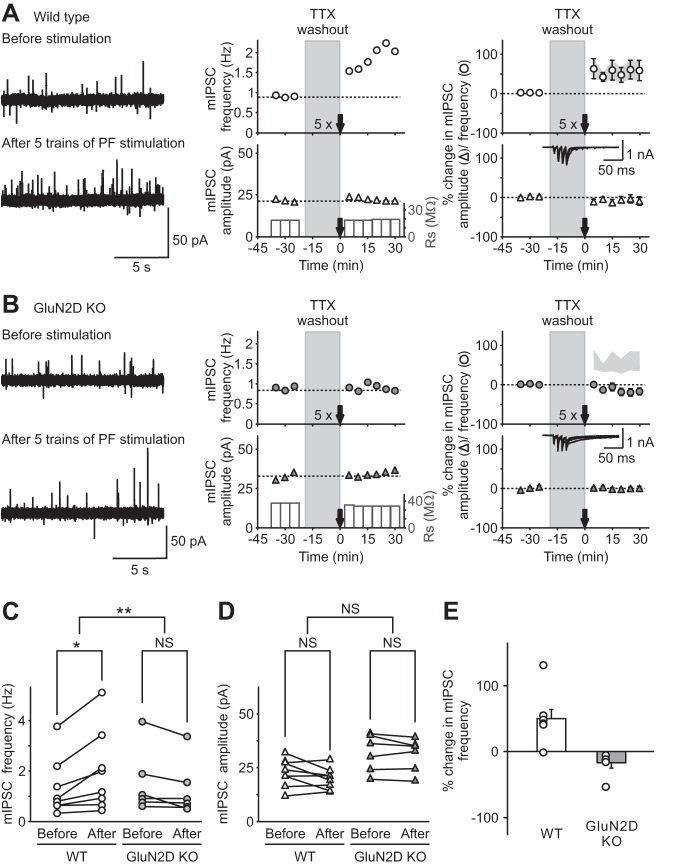
Fig. 3.
Genetic deletion of GluN2D subunits abolished long-term potentiation of inhibitory transmission (I-LTP) induced by threshold PF stimulation. A and B: miniature inhibitory synaptic currents (mIPSCs) were recorded in stellate cells in the presence of tetrodotoxin (TTX), and PFs were stimulated with 5 trains of 4 pulses at 100 Hz (5×) after washing out TTX for 20 min, in WT (A) and KO (B) mice. Left: representative traces of mIPSCs recorded at −30 mV (outward currents) before (top) and after (bottom) PF stimulation. Center: corresponding time course of mIPSC frequency (top) and amplitude (bottom). Right: group data shown as means ± SE showing the time course of mIPSC frequency (top) and amplitude (bottom) following PF stimulation in WT (open symbols; A) and in GluN2D KO mice (gray symbols; B; for comparison the light gray area shows the mean ± SE of mIPSC frequency after PF stimulation in WT mice). Insets: representative recordings at −60 mV during PF stimulation in stellate cells. Five trains of PF stimulation were sufficient to trigger I-LTP in stellate cells from WT mice (n = 8), but failed to induce a change in mIPSCs in GluN2D KO mice (n = 6). Therefore, NMDA receptors that contain GluN2D subunits mediate the induction of I-LTP. C and D: group data showing mIPSC frequency (C) and amplitude (D) of individual cells before (15 min average before TTX washout) and after (average 15–30 min after stimulation) PF stimulation in WT (left) and GluN2D KO (right) mice. Only mIPSC frequency from WT animals increased following 5 trains of PF stimulation. E: group data showing that 5 trains of PF stimulation increased mIPSC frequency in individual cells from WT, but not GluN2D KO, mice. *P < 0.05. **P < 0.01. NS, nonsignificant.