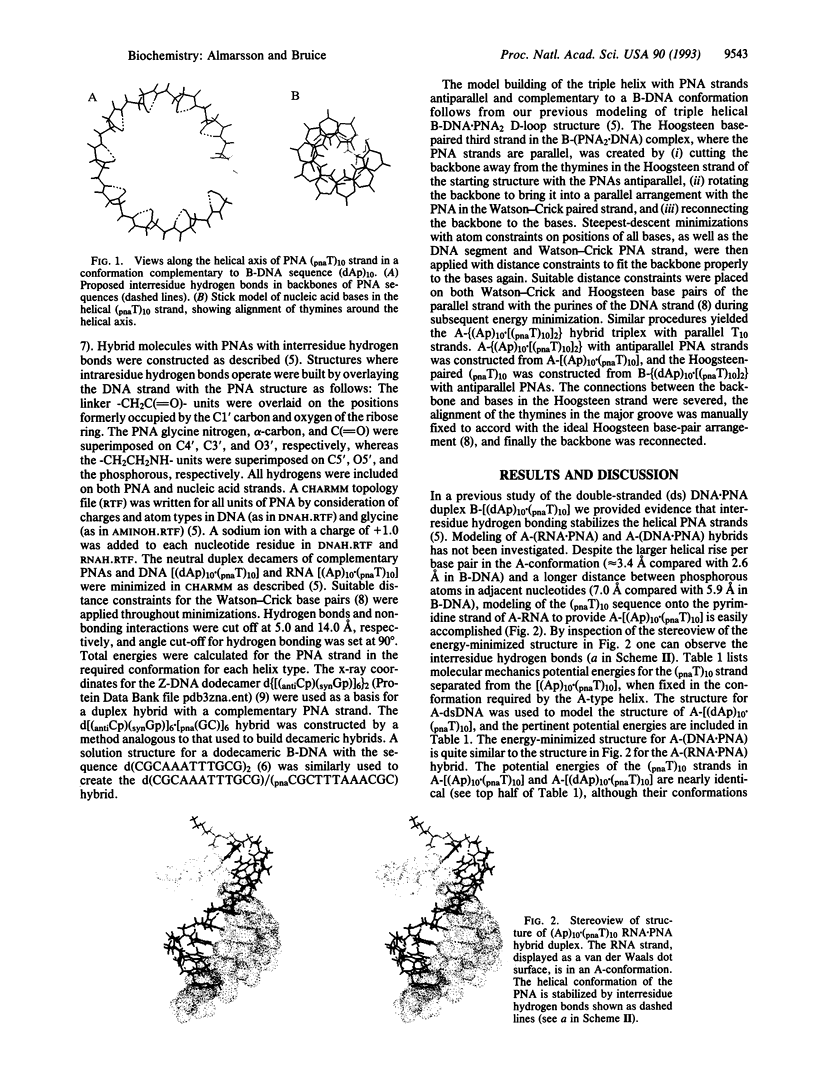
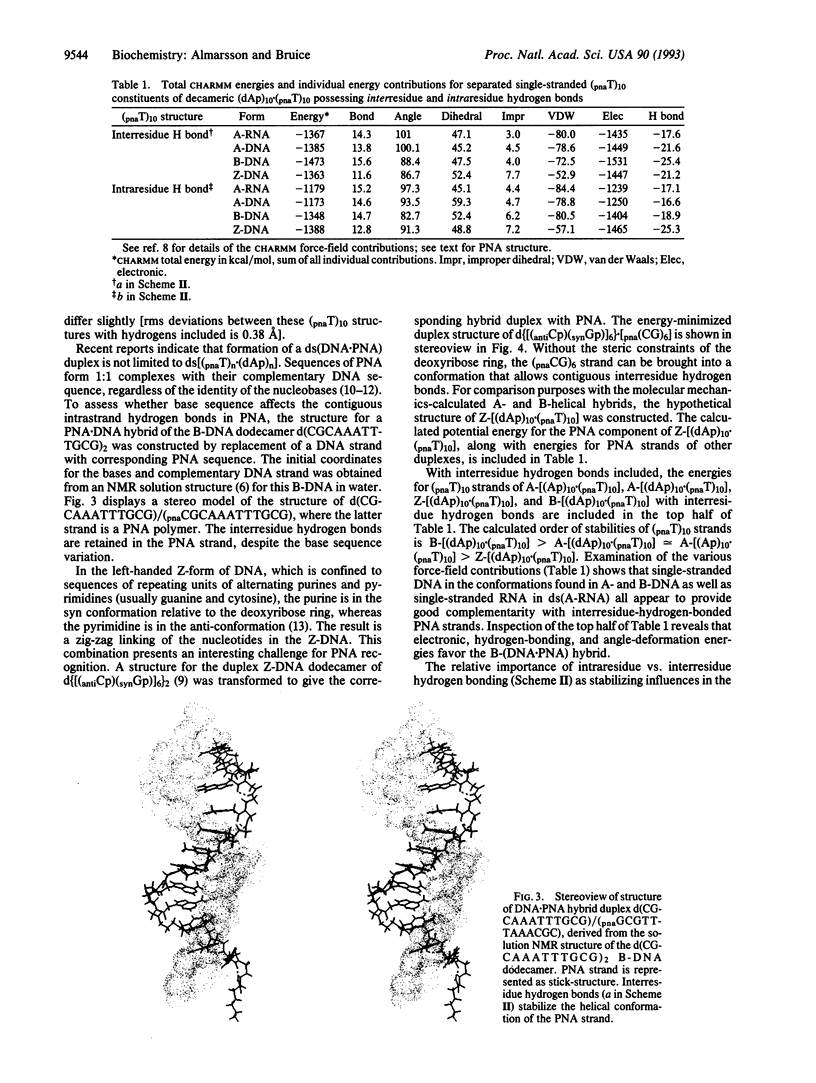
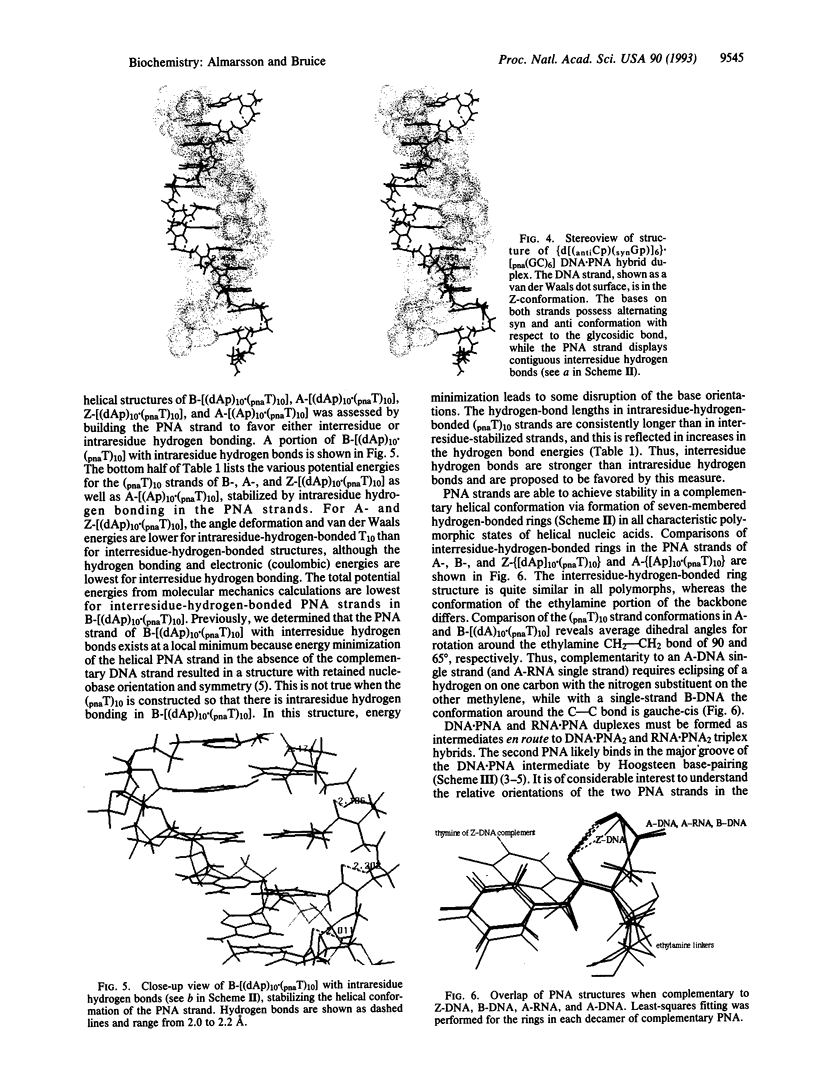
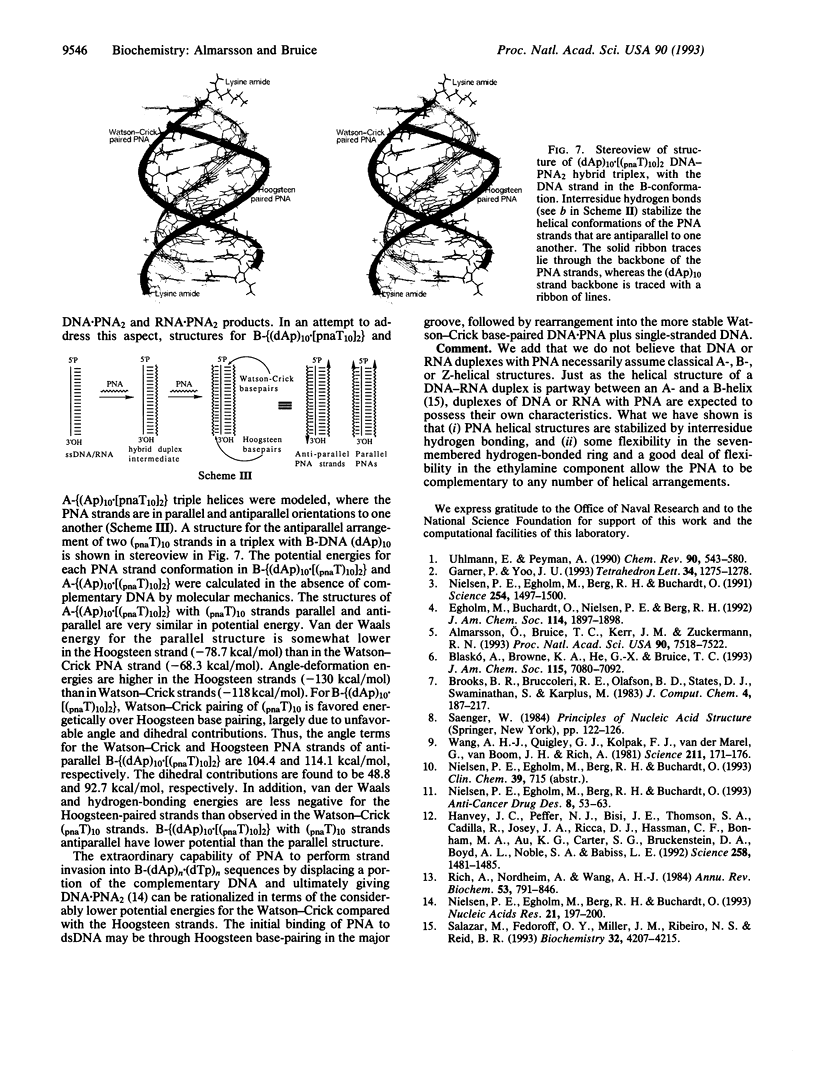
Abstract
Two hydrogen-bonding motifs have been proposed to account for the extraordinary stability of polyamide "peptide" nucleic acid (PNA) hybrids with nucleic acids. These interresidue- and intraresidue-hydrogen-bond motifs were investigated by molecular mechanics calculations. Energy-minimized structures of Watson-Crick base-paired decameric duplexes of PNA with A-, B-, and Z-DNA and A-RNA polymorphs indicate that the inherent stability of the complementary PNA helical structures is derived from interresidue, rather than from intraresidue, hydrogen bonds in all hybrids studied. Intraresidue-hydrogen-bond lengths are consistently longer than interresidue hydrogen bonds. Helical strand stability with interresidue hydrogen bond stabilization follows the order: B-(DNA.PNA) > A-(DNA.PNA) congruent to A-RNA.PNA > Z-(DNA.PNA). In the triplex hybrids A-(RNA.PNA2) and B-(DNA.PNA2), differences between stabilities of the two decamers of thyminyl PNA with lysine amide attached to the C terminus (pnaT)10 strands are small. The Hoogsteen (pnaT)10 strands are of slightly higher potential energy than are the Watson-Crick (pnaT)10 strands. Antiparallel arrangement of PNAs in the triplex is slightly favored over the parallel arrangement based on the calculations. Examination by molecular mechanics of the PNA.DNA analogue of the NMR-derived structure for the B-double-stranded DNA dodecamer d(CG-CAAATTTGCG)2 in solution suggests that use of all bases of the genetic alphabet should be possible without loss of the specific interresidue-hydrogen-bonding pattern within the PNA strand.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almarsson O., Bruice T. C., Kerr J., Zuckermann R. N. Molecular mechanics calculations of the structures of polyamide nucleic acid DNA duplexes and triple helical hybrids. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7518–7522. doi: 10.1073/pnas.90.16.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanvey J. C., Peffer N. J., Bisi J. E., Thomson S. A., Cadilla R., Josey J. A., Ricca D. J., Hassman C. F., Bonham M. A., Au K. G. Antisense and antigene properties of peptide nucleic acids. Science. 1992 Nov 27;258(5087):1481–1485. doi: 10.1126/science.1279811. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Peptide nucleic acids (PNAs): potential antisense and anti-gene agents. Anticancer Drug Des. 1993 Feb;8(1):53–63. [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Sequence specific inhibition of DNA restriction enzyme cleavage by PNA. Nucleic Acids Res. 1993 Jan 25;21(2):197–200. doi: 10.1093/nar/21.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991 Dec 6;254(5037):1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Salazar M., Fedoroff O. Y., Miller J. M., Ribeiro N. S., Reid B. R. The DNA strand in DNA.RNA hybrid duplexes is neither B-form nor A-form in solution. Biochemistry. 1993 Apr 27;32(16):4207–4215. doi: 10.1021/bi00067a007. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]



