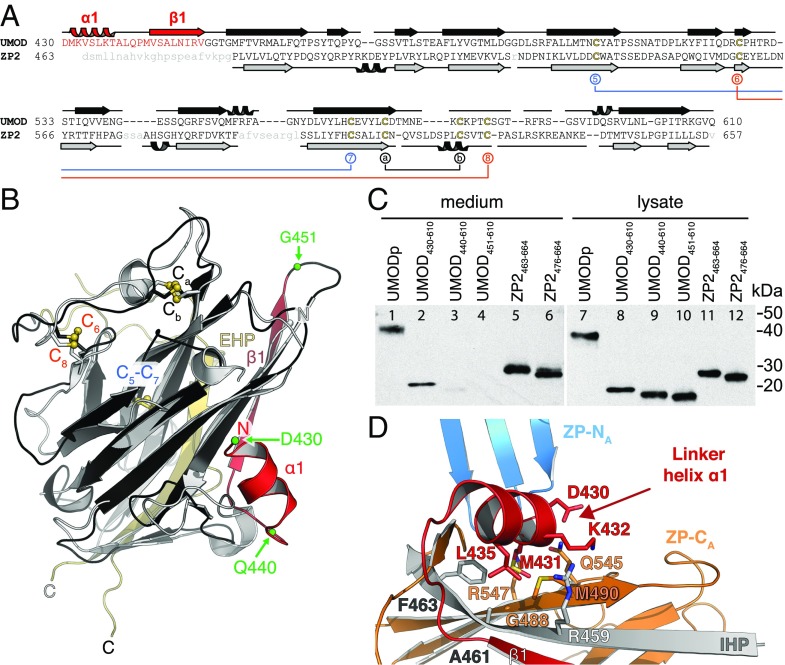
Fig. 4.
The structured ZP-N/ZP-C linker of UMOD is essential for ZP-C secretion and orients ZP-N relative to ZP-C. (A) Structure-based sequence alignment of UMOD and ZP2 ZP-Cs. The UMOD ZP-N/ZP-C linker is colored red, and ZP2 disordered residues are shown in lowercase gray. Disulfide bonds are colored as in Fig. 3B. (B) Structure comparison of the ZP-C domains of UMOD (black/red) and ZP2 (gray). The ZP-N/ZP-C linker of UMOD (red) is visible in the electron density, whereas the linker of ZP2 is flexible and not observed. Green spheres indicate truncation sites of the UMOD constructs analyzed in C. A side-by-side representation of this superposition can be found in Fig. S7. (C) Anti-5His immunoblot of conditioned medium and lysate of cells expressing different truncations of UMOD and ZP2. (D) UMOD ZP-C–associated α1 and β1 determine the relative position of ZP-N and ZP-C through hydrophobic interactions. Colors are as in Fig. 2A.