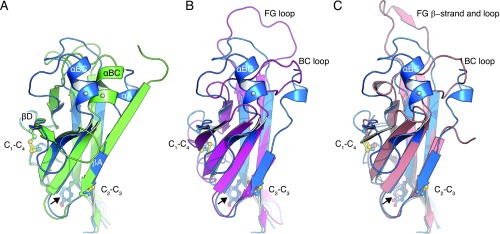
Fig. S4.
Structural comparison of ZP-N domains. (A) Conformational rearrangements of the ZP-N domains of UMOD molecules A (blue) and B (green). A coiled segment (UMOD residues 323–332) in molecule A follows the EGF IV domain, whereas the same segment forms a β-strand in molecule B. In the presence of strand αA, helix αBC folds over toward strand βD. The different conformation adopted by residues 323–332, as well as the concomitant changes in the loops connecting βB/βC, βD/βE, and βF/βG, are most likely due to the flexibility of mMBP and EGF IV in molecule B (Fig. S2A). The invariant Tyr implicated in filament formation in TECTA is indicated by a black arrow. Cys are displayed in a ball-and-stick representation and labeled according to their connectivity. C6′, which staples the EGF IV domain to the ZP-N domain (omitted for clarity; Fig. 2B), is indicated by a gray sphere in αBC. (B) Superposition of the ZP-NA domain of UMOD (blue) and the ZP-N domain of mouse ZP3 (PDB ID code 3D4G; magenta), with rmsd = 2.6 Å. UMOD lacks the large FG loop of ZP3. (C) Superposition of the ZP-NA domain of UMOD (blue) and the ZP-N domain of chicken ZP3 (PDB ID code 3NK4; salmon), with rmsd = 2.0 Å.