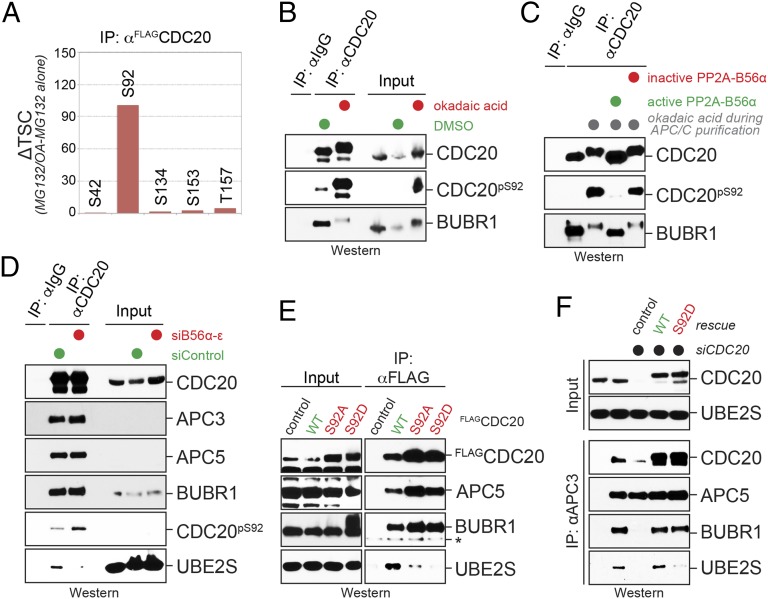
Fig. 4.
PP2AB56 regulates Cdc20 phosphorylation to control Ube2S recruitment to the APC/C. (A) Ser92 in Cdc20 is a phosphatase-sensitive site during prometaphase. FLAGCdc20 was purified from prometaphase cells in the presence or absence of okadaic acid, and the abundance of phosphorylated peptides was determined by mass spectrometry. (B) Phosphatase inhibition increases phosphorylation of Cdc20S92, as determined by Western blot analysis using a phospho-S92 specific antibody. Endogenous Cdc20 was purified from prometaphase extracts using monoclonal αCdc20-antibodies, and okadaic acid was added as indicated. (C) PP2AB56α targets Ser92 of Cdc20 in vitro. Cdc20 was purified from prometaphase extracts treated with okadaic acid, it was and incubated with purified PP2AB56α or with PP2AB56 that had been inactivated with okadaic acid. Phosphorylation of Cdc20 was analyzed by Western blot analysis using the pS92-specific antibody. (D) PP2AB56 targets Ser92 of Cdc20 in cells. HeLa cells were depleted of all B56 substrate targeting factors using validated siRNAs. After cells were synchronized in prometaphase, Cdc20 was affinity-purified, and Ser92 phosphorylation and Ube2S binding were measured by Western blot analysis. (E) A phosphomimetic mutation in Cdc20-Ser92 obliterates binding to Ube2S, but not the MCC. Wild type (WT), Ser92Ala, or Ser92Asp mutants of Cdc20 were immunoprecipitated from lysates of prometaphase cells and analyzed for Ube2S, APC/C, or MCC binding (as indicated by BubR1) by Western blot analysis. The asterisk marks a nonspecific band on the BubR1 Western blot. (F) Cdc20 phosphorylation prevents delivery of Ube2S to the APC/C. Cells were depleted of endogenous Cdc20 and reconstituted with either WT or phosphomimetic Cdc20S92D. APC/C was purified with αAPC3 antibodies and analyzed for bound proteins by Western blot analysis.