Cystic fibrosis (CF) is an autosomal recessive disease caused by genetic mutations in the CF transmembrane conductance regulator (CFTR), an anion transporter normally expressed in secretory glands of the lungs, pancreas, digestive tract, liver, skin, and reproductive organs. When functioning properly, the CFTR protein conducts intracellular chloride anions across an epithelial cell membrane into the extracellular space. In CF, a dysfunctional or absent CFTR protein results in the production of abnormally thick, sticky mucus in the respiratory tract, as well as impaired secretion of bodily fluids, like digestive enzymes, bile, sweat, tears, and semen. Although the clinical manifestations of CF are protean, most people with two abnormal CFTR alleles suffer some degree of digestive and respiratory disease and, particularly in males, infertility; currently, in the developed world, the median life expectancy in CF is around 40 y (1). CFTR gene defects are particularly common among persons of European heritage; ∼1 in 30 non-Hispanic whites is a carrier of a mutant CFTR allele, and 1 of every 2,300 white infants is born with CF (2).
One of the dreaded complications of CF lung disease is infection with Pseudomonas aeruginosa, which is an independent risk factor for excess morbidity and mortality in children with CF (3). In PNAS, Hendricks et al. (4) explore respiratory virus infection as a potential facilitator of P. aeruginosa acquisition in the CF airway.
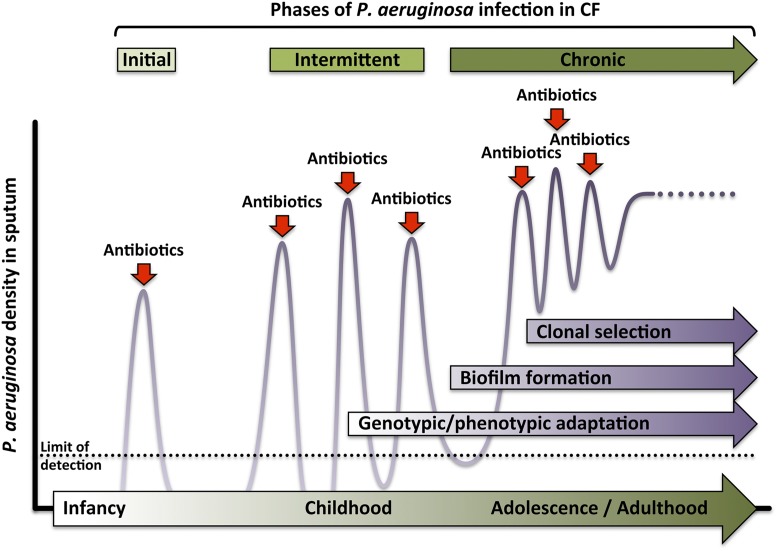
Environmental isolates of P. aeruginosa are responsible for initial infection in infancy or early childhood, and subsequent transient infections are common in CF (Fig. 1). However, over time P. aeruginosa undergoes genetic adaptations to the CF airway, including increased production of alginate, an exopolysaccharide that confers a slimy mucoid coat; decreased synthesis of flagellin, a protein that enables bacterial movement and navigation; and modifications to the cell wall component lipopolysaccharide (LPS). In general, these genetic modifications decrease the virulence of the organism but enhance its ability to survive in the infected host. The mucoid alginate coating protects the bacterium from phagocytosis, and alterations in flagellin and LPS, potent Toll-like receptor ligands, prevent induction of protective innate immune responses (3, 5). These genetic adaptations occur independently in different isolates over time; however, eventually, clonal selection of a specific genotype ensues, which then predominates in the chronic phase of infection (3, 5, 6).
Fig. 1.
Typical time course of P. aeruginosa colonization of the CF respiratory tract. Initial acquisition of a wild-type environmental isolate of P. aeruginosa is treated with antibiotics and eradicated. During subsequent intermittent infections, genetic adaptations to the CF airway result in impaired bacterial clearance. These adaptations include: a transition to a biofilm mode of growth, production of a mucoid coating to elude phagocytosis, altered expression of virulence factors like flagellin and LPS, and enhanced resistance to antibiotics. Eventually a dominant genotypic clone emerges, which continues to adapt to its host.
During persistent infection, P. aeruginosa also transitions to a communal mode of growth in biofilm, a sticky matrix of proteins, polysaccharides, and nucleic acids, secreted by surface-adherent bacteria, within which they replicate. Biofilm formation confers structural integrity to the bacterial colony and protects it from host defenses and antibiotics alike, in part by blocking their diffusion into the biofilm interior. Antibiotic concentrations of 1,000–10,000 times greater than those needed to kill a free-floating bacterium are required to kill bacteria in biofilms (7); thus, during repeated or prolonged antibiotic therapy, drug-resistant genotypes can be selected in biofilm-embedded bacteria exposed to subinhibitory antibiotic concentrations. Current practice favors early, aggressive treatment of P. aeruginosa infections in children with CF, with a goal of delaying the onset of chronic colonization and the clinical complications that follow (1, 5). In the past decade, the percentage of CF patients infected with P. aeruginosa has declined overall; however, ∼20% of adults with CF are colonized with multidrug-resistant isolates, likely resulting from cumulative antibiotic exposures (1).
Epidemiological studies have linked wintertime (8), and viral respiratory tract infections in particular (9, 10), with the acquisition of P. aeruginosa by CF patients. Viral-bacterial synergism in human disease has been well described between influenza virus and bacterial species that cause pneumonia, particularly Streptococcus pneumoniae and Staphylococcus aureus (11, 12). Previous research demonstrated that antecedent respiratory syncytial virus (RSV) infection promotes the adherence of P. aeruginosa to lung epithelium in vitro (13) and enhances bacterial replication in mice in vivo (14).
In PNAS, Hendricks et al. (4) extend these observations mechanistically, describing a dysregulation of nutritional immunity—particularly iron homeostasis—that accompanies respiratory virus infection and promotes Pseudomonas biofilm growth. Because most microbes require iron for metabolism and replication, humans have evolved elaborate iron-withholding defenses to keep it from invading pathogens. Iron stores are scrupulously maintained, primarily by intracellular sequestration in complex with hemoglobin or ferritin. Free iron is scarce; what little there is becomes rapidly bound up by extracellular transferrin or lactoferrin. As a consequence, nearly all bacteria use iron-snatching counter-measures, such as erythrocyte-destroying hemolysins or siderophores that chelate iron with greater affinity than host storage proteins (15). In acute infection, P. aeruginosa produces a major siderophore, pyoverdine, which scavenges ferric iron and imparts a characteristic green tint to its colonies in culture. In this arms race for iron, CF confers a distinct disadvantage to the host side. Clinical data suggest that iron and iron-binding proteins are inherently elevated in the sputum of CF patients, regardless of their bacterial colonization status (16), and in vitro experiments imply that defective CFTR underlies this baseline dysregulation of iron homeostasis (17). Now, Hendricks et al. (4) show that a respiratory virus infection can further sabotage host iron reserve mechanisms, enabling the transition to a biofilm growth mode in concurrent P. aeruginosa infection.
In an immortalized human CF bronchial epithelial cell line, homozygous for the CFTR mutation ΔF508, Hendricks et al. (4) demonstrate that infection with RSV, human rhinovirus 14, or human adenovirus 5 promotes biofilm formation upon subsequent P. aeruginosa infection. RSV-induced biofilm development was also observed in primary bronchial epithelial cells from both healthy donors and those with CF. The authors go on to show that Pseudomonas biofilm formation on CF epithelial cells is enhanced by RSV-induced type III IFN secretion at the apical cell membrane, accompanied by apical release of both iron and transferrin. Finally, in a neonatal mouse model, they demonstrate that levels of both iron and transferrin are higher in bronchoalveolar lavage fluid (BALF) from RSV-infected pups, compared with that from mock infections, and that BALF from the RSV-infected mice also supports in vitro P. aeruginosa biofilm formation. From these data, the authors suggest that the host innate immune response to viral respiratory infection, particularly a dysregulation of iron homeostasis, creates a microenvironment particularly favorable for biofilm production by P. aeruginosa.
Other respiratory pathogens—particularly S. aureus and Haemophilus influenzae—are commonly acquired by children and adolescents with CF. However, as CF patients age, Pseudomonas persists and predominates in the lung, often to the exclusion of other bacteria. Approximately two-thirds of all adults with CF are chronically colonized with P. aeruginosa (1). Why this occurs with P. aeruginosa in particular, and less so with the iron-requiring, biofilm-producing S. aureus, is not clear. Interestingly, Hendricks et al. (4) observed that RSV infection promotes P. aeruginosa biofilm growth on both primary CF and non-CF human bronchial epithelial cells. Although CFTR mutations are independently associated with
In PNAS, Hendricks et al. explore respiratory virus infection as a potential facilitator of P. aeruginosa acquisition in the CF airway.
excess iron secretion (16, 17), BALF from RSV-infected BALB/cJ mouse pups, which express wild-type CFTR, was also enriched in iron and able to promote in vitro biofilm formation by P. aeruginosa. Thus, respiratory virus-induced dysregulation of iron homeostasis appears to occur regardless of host CFTR genotype. Perhaps the iron-releasing effects of CFTR dysfunction and RSV infection are additive during acute infection with P. aeruginosa, promoting biofilm formation and thus persistence in CF airways in particular. However, viral respiratory infections do not appear to affect the presence or density of Pseudomonas in the sputum of CF patients already chronically colonized with it (18, 19).
If respiratory virus infections do promote P. aeruginosa acquisition in CF, the question arises as to what to do about it. As every parent knows, viral respiratory infections are all too common in childhood, with or without CF. RSV infects most infants during their first year of life, with nearly all children infected at least once by the age of 2 y (20). We lack vaccines or antiviral treatments for most respiratory viruses; for RSV, a monoclonal antibody directed against the surface fusion (F) protein, palivizumab, is given monthly during RSV season to premature infants and others at high risk for severe RSV disease (20). However, very limited clinical data have as yet failed to show a benefit with palivizumab prophylaxis in preventing either RSV infection or Pseudomonas colonization in infants with CF (21, 22). More antiviral vaccines and therapeutics are clearly needed for all children; in the meantime, compounds that help sequester iron away from bacteria might have clinical application in CF, as Hendricks et al. suggest (4). Indeed, gallium, an iron mimetic that is taken up by P. aeruginosa but is metabolically unable to substitute for it, is bactericidal in animal models and is in clinical trials in CF (23). Ultimately, in the absence of vaccines against the myriad of respiratory viruses that children face, antimicrobial therapies that give the host side an edge in the arms race for iron may help to level the battlefield for patients with CF.
Acknowledgments
The author’s research is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH/NIAID), Grants R01 AI110703-01 and R56 AI117287-01, and by the NIH/NIAID Centers for Excellence in Influenza Research and Surveillance, Contract HHSN272201400008C.
Footnotes
The author declares no conflict of interest.
See companion article on page 1642.
References
- 1.Cystic Fibrosis Foundation Patient Registry . 2014 Annual Data Report. Cystic Fibrosis Foundation; Bethesda, MD: 2015. [Google Scholar]
- 2.Brennan ML, Schrijver I. Cystic fibrosis: A review of associated phenotypes, use of molecular diagnostic approaches, genetic characteristics, progress, and dilemmas. J Mol Diagn. 2016;18(1):3–14. doi: 10.1016/j.jmoldx.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld M, Ramsey BW, Gibson RL. Pseudomonas acquisition in young patients with cystic fibrosis: Pathophysiology, diagnosis, and management. Curr Opin Pulm Med. 2003;9(6):492–497. doi: 10.1097/00063198-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Hendricks MR, et al. Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proc Natl Acad Sci USA. 2016;113:1642–1647. doi: 10.1073/pnas.1516979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuart B, Lin JH, Mogayzel PJ., Jr Early eradication of Pseudomonas aeruginosa in patients with cystic fibrosis. Paediatr Respir Rev. 2010;11(3):177–184. doi: 10.1016/j.prrv.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith EE, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103(22):8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnanadhas DP, et al. Successful treatment of biofilm infections using shock waves combined with antibiotic therapy. Sci Rep. 2015;5:17440. doi: 10.1038/srep17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen HK, Høiby N. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax. 1992;47(2):109–111. doi: 10.1136/thx.47.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen NT, et al. Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma—Possible synergism with Pseudomonas aeruginosa. Acta Paediatr Scand. 1981;70(5):623–628. doi: 10.1111/j.1651-2227.1981.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 10.Collinson J, et al. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax. 1996;51(11):1115–1122. doi: 10.1136/thx.51.11.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19(3):571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rynda-Apple A, Robinson KM, Alcorn JF. Influenza and bacterial superinfection: Illuminating the immunologic mechanisms of disease. Infect Immun. 2015;83(10):3764–3770. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Ewijk BE, et al. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res. 2007;61(4):398–403. doi: 10.1203/pdr.0b013e3180332d1c. [DOI] [PubMed] [Google Scholar]
- 14.de Vrankrijker AM, et al. Respiratory syncytial virus infection facilitates acute colonization of Pseudomonas aeruginosa in mice. J Med Virol. 2009;81(12):2096–2103. doi: 10.1002/jmv.21623. [DOI] [PubMed] [Google Scholar]
- 15.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghio AJ, et al. Iron accumulates in the lavage and explanted lungs of cystic fibrosis patients. J Cyst Fibros. 2013;12(4):390–398. doi: 10.1016/j.jcf.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Moreau-Marquis S, et al. The DeltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L25–L37. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin M, et al. Acute effects of viral respiratory tract infections on sputum bacterial density during CF pulmonary exacerbations. J Cyst Fibros. 2015;14(4):482–489. doi: 10.1016/j.jcf.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esther CR, Jr, Lin FC, Kerr A, Miller MB, Gilligan PH. Respiratory viruses are associated with common respiratory pathogens in cystic fibrosis. Pediatr Pulmonol. 2014;49(9):926–931. doi: 10.1002/ppul.22917. [DOI] [PubMed] [Google Scholar]
- 20.Committee on Infectious Diseases, American Academy of Pediatrics . Respiratory syncytial virus. In: Kimberlin DW, editor. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th Ed American Academy of Pediatrics; Elk Grove Village, IL: 2015. [Google Scholar]
- 21.Robinson KA, Odelola OA, Saldanha IJ. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database Syst Rev. 2014;5:CD007743. doi: 10.1002/14651858.CD007743.pub5. [DOI] [PubMed] [Google Scholar]
- 22.Linnane B, Kiernan MG, O’Connell NH, Kearse L, Dunne CP. Anti-RSV prophylaxis efficacy for infants and young children with cystic fibrosis in Ireland. Multidiscip Respir Med. 2015;10:32. doi: 10.1186/s40248-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ClinicalTrials.gov (2015) A pharmacokinetic and safety study of IV gallium nitrate (ganite) in cystic fibrosis patients (National Library of Medicine, Bethesda, MD). Available at https://clinicaltrials.gov/ct2/show/NCT01093521. Accessed January 13, 2016.