Figure 3.
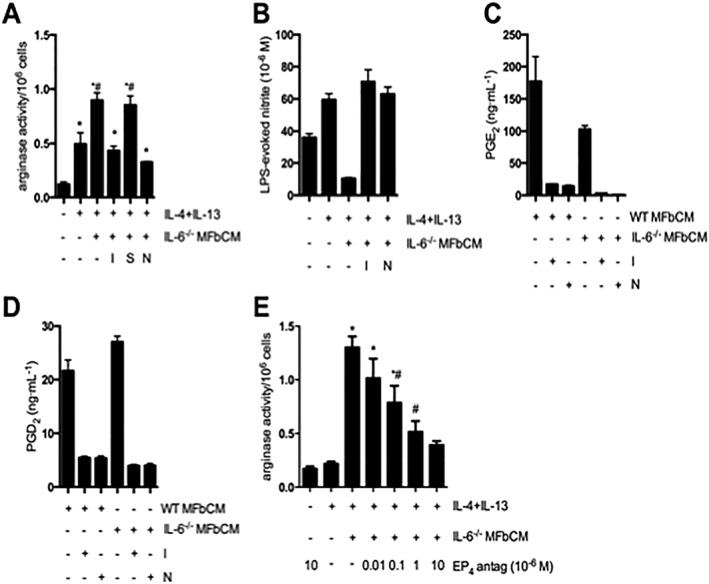
COX‐2‐derived products from dermal myofibroblasts enhance AAM function. (A–B) Myofibroblast conditioned medium from IL‐6−/− myofibroblasts treated with indomethacin (COX‐1 and 2 inhibitor, 1 μM), SC‐560 (COX‐1 inhibitor, 1 μM) and NS‐398 (COX‐2 inhibitor, 20 μM) at 0 and 24 h was used to stimulate macrophages (+IL‐4 + IL‐13). MFbCM from myofibroblasts exposed to indomethacin (I) and NS‐398 (N), but not SC‐560 (S), lost their ability to increase arginase activity in IL‐4 + IL‐13‐treated macrophages. MFbCM from myofibroblasts exposed to indomethacin (I) and NS‐398 (N), lost their ability to suppress nitric oxide production in IL‐4 + IL‐13‐treated macrophages (A; n = 5–11 except negative control where n = 3; B; n = 3;). (C–D) Production of PGE2 and PGD2 by wild type (WT) and IL‐6−/− myofibroblasts was suppressed by treatment with indomethacin and NS‐398 (n = 3,). (E) Enhanced arginase activity in IL‐4 + IL‐13 + MFbCM‐treated macrophages was dose‐dependently inhibited by the EP4 receptor antagonist, L161982 (EP4 antag) (n = 6, except IL‐4 + IL‐13 + MFbCM + EP4 antag (10 × 10−6 M) where n = 3). Data are mean ± SEM; * P < 0.05 versus untreated macrophages, #P < 0.05 versus IL‐4 + IL‐13 + IL‐6−/− MFbCM; anova followed by Tukey's post hoc test.
