Summary
Cell-cell fusion plays essential roles during fertilization and organogenesis. Previous studies in C. elegans led to the identification of the eukaryotic fusion protein (EFF-1 fusogen), which has structural homology to class II viral fusogens. Transcriptional repression of EFF-1 ensures correct fusion fates, and overexpression of EFF-1 results in embryonic lethality. EFF-1 must be expressed on the surface of both fusing cells; however, little is known regarding how cells regulate EFF-1 surface exposure. Here, we report that EFF-1 is actively removed from the plasma membrane of epidermal cells by dynamin- and RAB-5-dependent endocytosis and accumulates in early endosomes. EFF-1 was transiently localized to apical domains of fusion-competent cells. Effective cell-cell fusion occurred only between pairs of cell membranes in which EFF-1 localized. Downregulation of dynamin or RAB-5 caused EFF-1 mislocalization to all apical membrane domains and excessive fusion. Thus, internalization of EFF-1 is a safety mechanism preventing excessive cell fusion.
Graphical Abstract
Highlights
-
•
The fusion protein EFF-1 is targeted to early endosomes
-
•
Dynamin and RAB-5 downregulate EFF-1 in C. elegans embryos
-
•
Transient and dynamic localization of EFF-1 to apical cell membranes mediates fusion
-
•
Prevention of EFF-1 endocytosis induces excessive cell fusion
Smurova and Podbilewicz find that RAB-5 and dynamin-mediated endocytosis removes the fusogen EFF-1 from the plasma membrane and serves as a negative regulator of cell-cell fusion in C. elegans embryos. Thus, dynamic and transient localization of EFF-1 on the apical plasma membranes is sufficient to merge neighboring cells.
Introduction
Cell-to-cell fusion initiates the process of sexual reproduction and, following fertilization, sculpts organs such as muscle, bone, eye lens, and placenta in the developing organism (Aguilar et al., 2013). Cell fusion is also involved in inflammation, regeneration, wound healing, and cancer (Losick et al., 2013, Medvinsky and Smith, 2003, Oren-Suissa and Podbilewicz, 2010, Rizvi et al., 2006). Nevertheless, little is known about mechanisms that regulate cell fusion (Chen et al., 2007, Podbilewicz, 2014). In the nematode Caenorhabditis elegans, one-third of all somatic cells fuse during development, making this organism attractive for studying cell fusion (Gattegno et al., 2007, Podbilewicz and White, 1994, Shinn-Thomas and Mohler, 2011). The first identified eukaryotic fusogen, the C. elegans epithelial fusion failure 1 (EFF-1), mediates fusion of cells in the hypodermis (skin), pharynx, and vulva (Mohler et al., 2002). Ectopic expression of EFF-1 can induce fusion of cells that normally do not fuse both in C. elegans and in heterologous cells grown in culture (Avinoam et al., 2011, Podbilewicz et al., 2006, Shemer et al., 2004). Fusion of these cells requires EFF-1 expression in both fusing partners (Avinoam et al., 2011, Kim et al., 2015, Podbilewicz et al., 2006, Shilagardi et al., 2013). Because EFF-1 is a potent fusogen and its ectopic expression induces embryonic lethality, it must be regulated in space and time. Different genetic pathways including Engrailed/CEH-16, GATA factors, Hox, Notch, RTK, and Wnt signaling regulate eff-1 activity directly or indirectly (Alper and Kenyon, 2002, Brabin et al., 2011, Cassata et al., 2005, Fernandes and Sternberg, 2007, Kontani et al., 2005, Margalit et al., 2007, Pellegrino et al., 2011, Rasmussen et al., 2008, Shemer and Podbilewicz, 2002, Walser et al., 2006, Weinstein and Mendoza, 2013). However, very little is known about EFF-1 regulation at the protein level.
We aimed to understand which cellular mechanisms are involved in EFF-1 posttranslational regulation. The endocytic pathway controls numerous cellular processes including signaling pathways, epithelial polarity, cellular remodeling, synaptic transmission, cancer, and osteoclast and myoblast fusion (Chen et al., 2006, Fares and Greenwald, 2001, Grant and Hirsh, 1999, Leikina et al., 2013, Luga et al., 2012, Mellman and Yarden, 2013, Sato and Sato, 2013, Shin et al., 2014, Watanabe et al., 2013). Researchers have uncovered the role of actin, lipids, membrane curvature-modulating proteins, and dynamin in clathrin-dependent and -independent pathways of endocytosis (Kozlov et al., 2014, McMahon and Boucrot, 2011, Messa et al., 2014, Schmid et al., 2014). Rab proteins, small GTP-binding proteins of the Ras superfamily, control trafficking between organelles, including the ER, Golgi, plasma membrane, endosomes, and lysosomes (Grant and Donaldson, 2009, Mellman, 1996, Mizuno-Yamasaki et al., 2012). The Rab5 GTPase was shown to be a central regulator of the endolysosomal system as loss of Rab5 function caused a reduction in the number of endosomes and lysosomes and associated block of endocytosis (Zeigerer et al., 2012). However, little is known about membrane trafficking during developmental cell fusion.
Here, we show that endocytosis regulates homotypic EFF-1-mediated cell-cell fusion in C. elegans embryos. EFF-1 colocalizes with RAB-5 in early endosomes before and during fusion, whereas RAB-5 depletion results in EFF-1 mislocalization to the apical plasma membrane and induces ectopic fusion. EFF-1 localization at the apical plasma membrane is dynamic and transient due to its downregulation by dynamin- and RAB-5-dependent endocytosis. Membrane merger is initiated only when both apposing apical plasma membranes co-express EFF-1.
Results
EFF-1 Localizes to Intracellular Puncta
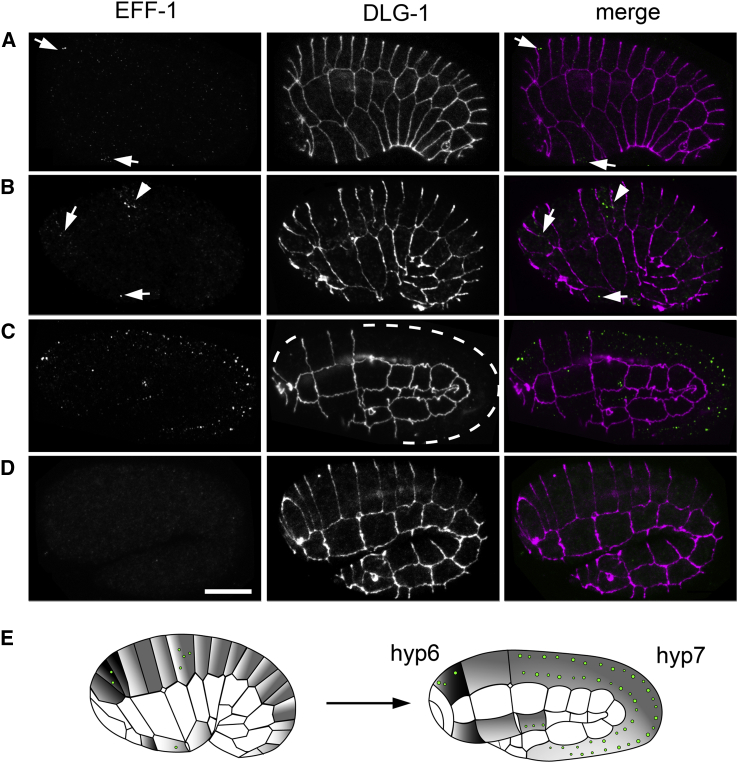
To uncover the expression pattern of the EFF-1 protein during development, its endogenous localization was followed by immunofluorescence with specific monoclonal antibodies against the extracellular domain of EFF-1 (Fridman, 2012; K. Fridman, C. Valansi, O. Avinoam, M. Oren, D. Pérez, C. Sánchez Espinel, Á. González-Fernández, A. Rotem, A. Harel, T. Krey, F.A. Rey, J.M. White, and B.P., unpublished data). EFF-1 was first detected at the bean stage within individual puncta in the dorsal and ventral hypodermal cells before fusion (n = 14; Figure 1A, arrows). Subsequently, EFF-1 appeared as punctate staining in the cytoplasm of hyp6 and hyp7 precursor cells at the comma stage (n = 18; Figure 1B, arrows). Following embryonic fusions, EFF-1 remained vesicular, and the number of EFF-1 puncta increased when cell fusion was nearly completed (n = 20; Figure 1C). EFF-1 puncta showed minor colocalization with apical cell junctions detected by anti-DLG-1 antibody (Figure 1B, arrowhead). We found that EFF-1 puncta were aligned along longitudinal lines lying parallel to the seam cells (Figure 1C). This arrangement might be dictated by the organization of the cytoskeleton in the syncytial hypodermal cells, where actin, intermediate filaments, and microtubules form bundles that run parallel to the seam cells (Figure S1). eff-1(ok1021)-null embryos at any stages did not show immunoreactivity, revealing the specificity of the monoclonal antibodies (Figure 1D). Thus, EFF-1 is expressed in puncta at the onset, during, and after hypodermal cell fusion in developing embryos (Figure 1E).
Figure 1.
EFF-1 Localizes to Intracellular Puncta
(A–C) EFF-1 expression pattern in the process of cell fusion was revealed by immunostaining with anti-EFF-1 monoclonal antibody (green) and anti-DLG-1 antibody (apical junctions, magenta). EFF-1 vesicular localization (arrows) at developmental stages prior to (A and B) and after epithelial cell fusion (C) in wild-type C. elegans embryos is shown. Colocalization of EFF-1 with apical junction is shown (arrowhead; B). Fused hyp6 and hyp7 syncytia are outlined with a white dashed line in (C).
(D) Immunostaining of eff-1(ok1021) mutant at 1.8-fold stage shows no EFF-1 expression, revealing the antibody specificity, and lack of cell fusion. The scale bar represents 10 μm.
(E) Schematics of embryonic epithelial cell fusions during elongation from comma (left) to 1.8-fold stage (right). Cell borders (junctions) are drawn with black lines; EFF-1 expression pattern is visualized as green puncta. Cells forming hyp6 and hyp7 syncytia are colored with dark gray and light gray, respectively.
Orientation of embryos in all figures is anterior, left and dorsal, top. See also Figure S1.
RAB-5 and DYN-1 RNAi Induce EFF-1 Plasma Membrane Accumulation
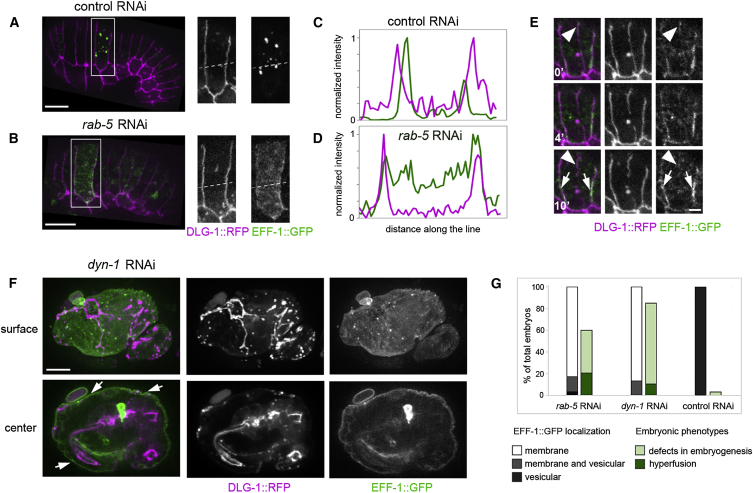
To follow the dynamics of EFF-1 expression in live embryos, we generated a transgenic strain carrying a fosmid-based reporter construct containing the entire EFF-1 locus fused to GFP, as well as ∼10 kb of upstream and downstream cis-regulatory regions (Sarov et al., 2012). The EFF-1::GFP fosmid was injected into the eff-1(hy21ts) mutant to prevent lethality induced by overexpression of EFF-1. Because EFF-1::GFP rescued the fusion failure mutant phenotype, we assumed that EFF-1::GFP was active and its expression level was similar to the endogenous EFF-1 level. Most of the EFF-1 protein localizes to intracellular puncta both before and during the fusion process (Figure 2A; Movies S1 and S2). To understand whether EFF-1 vesicular localization at steady state is dependent on rapid internalization from the plasma membrane, we hypothesized that reducing endocytic efficiency will result in localization of EFF-1 to the plasma membrane. We depleted rab-5, an essential regulator of endocytosis, using RNAi and followed EFF-1::GFP by live imaging. We found that rab-5 RNAi embryos showed enrichment of EFF-1::GFP in apical plasma membrane domain, rather than the bright organelles observed in the control embryos (Figures 2A–2D; Movies S3 and S4). In control embryos, there was only modest colocalization between EFF-1::GFP and DLG-1::RFP (Figures 2A and 2C). In contrast, in rab-5(RNAi), the intensity profile of EFF-1::GFP along a random line within the cell shows peaks overlapping with DLG-1::RFP peaks (Figure 2D), confirming the visual observation of enrichment of EFF-1 on apical junctions. Thus, EFF-1 is not present in apical cell membranes in the steady state. The overall level of EFF-1 was not changed; the average EFF-1::GFP intensity in control RNAi (70 ± 23 gray values/pixel; 17 cells from ten embryos) was similar to EFF-1::GFP intensity after rab-5 RNAi (69 ± 10 gray values/pixel; 23 cells from eight embryos). Our data suggest that, when rab-5 activity is reduced, EFF-1::GFP redistributes from intracellular vesicles to the plasma membranes.
Figure 2.
RAB-5 and DYN-1 RNAi Knockdown Induces EFF-1 Accumulation on the Apical Plasma Membrane
(A, B, and F) Live images of EFF-1::GFP (green) and apical junctions, DLG-1::RFP (magenta) in embryos treated with control RNAi (A), rab-5 RNAi (B), and dyn-1 RNAi (F). Right panel represents the magnified region of the inset area in (A) and (B).
(A) EFF-1::GFP is in puncta in control RNAi. See also Movies S1 and S2.
(B) EFF-1::GFP in rab-5 RNAi embryo appears diffuse on the cytoplasm in smaller vesicles and associated with the apical plasma membranes and junctions. See also Figure S2G and Movies S3 and S4.
(C) Intensity profile plotted along the line (white dashed line in A) of EFF-1::GFP (green line on the plot) and DLG-1::RFP (magenta line on the plot) shows no correlation.
(D) Intensity profiles plotted along dashed line from (B) represent correlation of EFF-1::GFP and DLG-1::RFP intensities on the cell junctions, showing the enrichment of EFF-1::GFP on the apical plasma membrane.
(E) Representative stages from time-lapse images of cell fusion under rab-5 RNAi showing EFF-1::GFP/DLG-1::RFP colocalization (arrows). Cell junction undergoing fusion is marked by an arrowhead. Note that EFF-1 dissociated from the cell junction at 10 min of fusion (arrowhead). Time in min is indicated. See also Movie S3.
(F) EFF-1::GFP apical plasma membrane expression (arrows) caused by dyn-1 RNAi is shown on the embryo surface focus (upper panels) and center focus (lower panels). Embryogenesis defects and hyperfusion are visualized by DLG-1::RFP pattern (Movie S5).
(G) EFF-1 localization and embryonic defects caused by rab-5 and dyn-1 downregulation. The first bar graph represents percentage of embryos showing EFF-1 localization with plasma membrane (white), plasma membrane together with vesicular puncta (gray), and vesicular expression (black). The second bar represents the percentage of embryos presenting defective embryogenesis (light green) and hyperfusion (dark green). Most rab-5(RNAi) and dyn-1(RNAi) embryos arrest at early embryogenesis before EFF-1 expression and before the time of hypodermal fusions (elongation/morphogenesis). Most embryos that escape the early arrest show hyperfusion and EFF-1 in apical plasma membranes. Mean of three independent experiments; n ≥ 20 embryos per experiment.
The scale bars represent 10 μm in (A), (B), and (F) and 2 μm in (E).
To determine the effect of rab-5 knockdown on the dynamics of EFF-1 at the plasma membrane during fusion, we followed EFF-1::GFP colocalization with the apical plasma membrane using the DLG-1::RFP reporter protein. We found that, when the apical junctions begin to disassemble in rab-5 RNAi embryos, EFF-1 is still weakly detected on cell membranes (Figure 2E, arrowhead, time 0’; Movie S3), but after 10 min of apical junction disassembly, EFF-1::GFP cannot be detected where the plasma membrane used to be (Figure 2E, arrowhead). Taken together, our results show that reduction in RAB-5 activity stabilizes EFF-1 localization at the apical plasma membrane of fusing cells, suggesting that RAB-5 is involved in the uptake of EFF-1 from the apical plasma membrane to endosomes.
To independently determine whether EFF-1 is indeed removed from the plasma membrane by endocytosis, we depleted a central endocytosis regulator, dynamin. DYN-1 is the only dynamin in C. elegans and is essential for embryogenesis (Clark et al., 1997). Significantly, in all surviving embryos that developed to the morphogenesis stages when fusion occurs (Figure 1), dyn-1 RNAi induced EFF-1::GFP mislocalization to the apical membrane of hypodermal cells (Figures 2F, 2G, and S2; Movie S5). These results support the hypothesis that EFF-1 localizes to endosomal organelles in the steady state. However, during embryonic morphogenesis, EFF-1 is continuously recycling between the apical plasma membrane and the endolysosomal system via receptor-mediated endocytosis. When internalization is blocked, EFF-1 mislocalizes to the apical plasma membrane.
RAB-5 and DYN-1 Control Cell Fusion
dyn-1 and rab-5 knockdown results in early embryonic arrest. Because most embryos arrest before EFF-1 expression and morphogenesis, we analyzed embryos that escape early arrest. dyn-1 RNAi treatments showed defects associated with ectopic fusion in 10%–20% of all the embryos (Figure 2G). The dyn-1 RNAi-induced hyperfusion phenotype was not observed in eff-1(hy21) embryos that lost the extrachromosomal EFF-1::GFP (n ∼ 100), demonstrating that ectopic fusion observed following dynamin downregulation is mediated by EFF-1, indicating that eff-1 is epistatic to dyn-1.
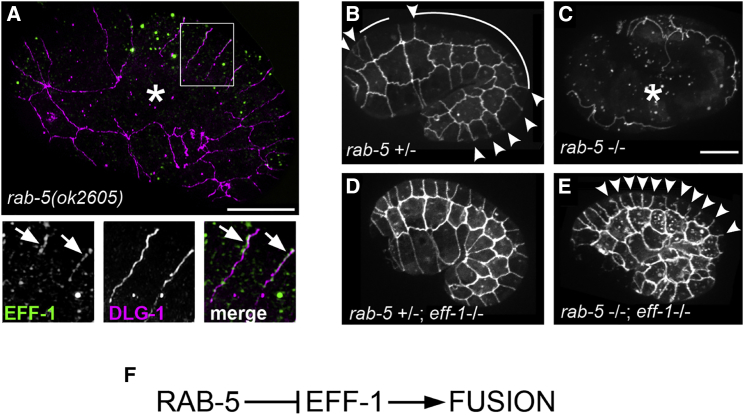
Like with dyn-1(RNAi), in rab-5(ok2605)-null mutant, endogenous EFF-1 was mislocalized to apical junctions (Figure 3A), consistent with the rab-5 RNAi effect on EFF-1::GFP localization (Figures 2D and 2E). Moreover, we found that 20% of the rab-5(ok2605) embryos that escape early lethality showed ectopic fusion phenotype (n = 35; Figure 3A, asterisk).
Figure 3.
Loss of rab-5 Function Induces eff-1-Mediated Ectopic Fusion
(A) Ectopic fusion (asterisk) and EFF-1 mislocalization to the plasma membrane (arrows) caused by rab-5(ok2605). Immunofluorescence with anti-EFF-1 (green) and anti-DLG-1 antibody (magenta) followed by SIM is shown. Magnifications of the inset region represent EFF-1 and DLG-1 fluorescence in separate channels and merged.
(B–E) Epistasis analysis between rab-5 and eff-1 reveals rab-5 as a negative regulator of eff-1. Fusion pattern was visualized by live imaging of junction marker DLG-1::RFP expressed in rab-5 and eff-1 single and double mutants.
(B) Heterozygous rab-5 mutant exhibits wild-type fusion. Fused cells are outlined with white stroke, junctions that will fuse later in embryogenesis (arrowheads).
(C) Hyperfusion induced by rab-5 homozygous mutation is shown (asterisk).
(D) No fusion in double rab-5(+/−); eff-1(−/−) mutant is shown.
(E) Fusion is blocked in double rab-5(−/−); eff-1(−/−) mutant, unfused junctions (arrowheads).
(F) Scheme of RAB-5-negative regulation of EFF-1 derived from the epistasis analysis. The scale bars represent 10 μm.
To find out whether the hyperfusion phenotype induced by rab-5 knockdown depends on eff-1 activity, we followed the fusion phenotype of double eff-1;rab-5 mutants. rab-5 heterozygous embryos showed normal fusion pattern (Figure 3B). In contrast, we observed excessive fusion in rab-5 homozygous embryos (Figure 3C, asterisk). We found that eff-1(hy21) mutants displayed suppressed cell fusion in both rab-5 hetero- and homozygous embryos (Figures 3D and 3E). Thus, eff-1 is epistatic to rab-5, suggesting that the hyperfusion induced by the deletion of rab-5 is eff-1 dependent. In other words, rab-5 inhibits the fusion-inducing activity of eff-1 (Figure 3F). We conclude that downregulation of dynamin or RAB-5 results in an increase in EFF-1 localization to the apical plasma membrane and hyperfusion in embryos that did not arrest early in embryogenesis (Figures 2 and S2).
To identify additional genes responsible for intracellular EFF-1 trafficking, localization, and function, we screened candidate genes for defects in cell fusion and EFF-1 localization using immunofluorescence and live imaging. Most trafficking mutants tested did not show differences compared with wild-type animals (Tables S2 and S3). As previously shown by Kontani et al. (2005), mutations in the vacuolar ATPase complex proteins caused hyperfusion in late stages of embryonic morphogenesis (Figure S3). Because mutations in the V-ATPase complex affect multiple endocytic trafficking pathways (Nishi and Forgac, 2002) and exocytosis of multivesicular bodies (Liégeois et al., 2006), it is difficult to distinguish which trafficking stages were involved in EFF-1-retarded hyperfusion.
EFF-1 Localizes to Early Endosomes
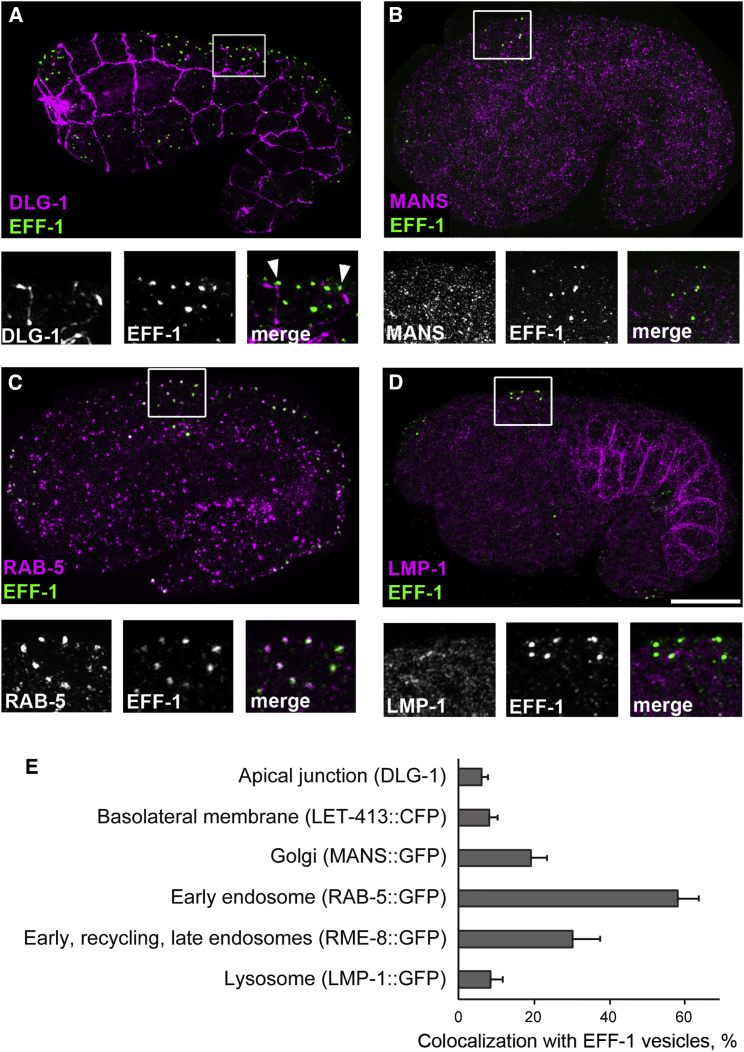
To identify the puncta where EFF-1 localizes, we performed colocalization studies using structured illumination microscopy (SIM). C. elegans embryos that express GFP-tagged cellular markers were immunostained with anti-GFP antibody to detect intracellular membrane compartments and with anti-EFF-1 antibody to localize endogenous EFF-1. Colocalization was quantified as percentage of endogenous EFF-1 puncta that overlapped with each of the anti-GFP antibodies on superresolution 3D images (see Experimental Procedures). Only 6% of EFF-1 puncta were localized to the region of apical junctions, whereas 13% were associated with apical junctions within 200 nm distance (Figure 4A). EFF-1 was enriched in structures that were positive for the early endosome marker RAB-5::GFP (58% of colocalization; n = 21 embryos; Figures 4C and 4E; Table S1). Thirty percent of EFF-1 puncta colocalized with the general endosomal marker RME-8 (early, late endosome, and multivesicular body; Figures 4E, S4E, and S4F; Table S1), supporting EFF-1 presence in RAB-5-positive early endosomes. EFF-1 also showed significant colocalization with the Golgi marker MANS::GFP (19%), possibly due to secretory sorting and recycling between endosomes and the Golgi (Figures 4B and 4E). Nine percent of EFF-1 puncta colocalized with the lysosomal marker LMP-1::GFP (Figures 4D and 4E), suggesting that EFF-1 is also transported to lysosomes where it is probably degraded. EFF-1 puncta colocalized less than 5% with most other markers examined (RAB-10::GFP, RME-1::GFP, RAB-11::GFP, RAB-7::GFP, ALX-1::GFP, LGG-1::GFP, and VHA-5::GFP; Table S1). Data sets for these markers were not statistically different from each other and probably represent the background of the measurements. Thus, wild-type endogenous EFF-1 is detected mainly in RAB-5 early endosomes and is only transiently associated with the apical domains of the plasma membrane where it acts to fuse cells.
Figure 4.
EFF-1 Localization to Intracellular Compartments
(A) The localization of EFF-1 revealed by an anti-EFF-1 monoclonal antibody (green) is compared with the apical junction (anti-DLG-1 antibody, magenta) using superresolution microscopy (SIM).
(B–D) EFF-1 colocalization with stably expressed GFP-tagged markers of different membrane-bound organelles, detected with anti-GFP antibodies, magenta: Golgi complex, MANS::GFP (B); early endosomes, RAB-5::GFP (C), and lysosomes, LMP-1::GFP (D). Lower panel represents inset areas enlarged and shown in separate channels and merged.
(E) Quantification of EFF-1 colocalization with different markers represents the ratio of EFF-1 puncta that overlay the puncta of indicated marker (number of colocalized EFF-1 puncta/total number of EFF-1 puncta as percentage). Bars represent mean percentage of colocalization calculated in 5–20 embryos (100–1,000 cells) ± SEM. The colocalization above 5% is shown in the graph. The full list of intracellular markers tested, number of puncta, and number of embryos per marker are shown in Table S1. See also Figure S4. The scale bar represents 10 μm.
We found that, in a mixed population of embryos expressing EFF-1, 58% of the puncta colocalized with RAB-5 (Figure 4E). To determine whether EFF-1/RAB-5 colocalization changes over time of fusion, we measured their colocalization during distinct stages in embryonic morphogenesis. When hypodermal cell fusions are in progress (1.5-fold stage of elongation), we found that 45% of EFF-1 puncta colocalize with RAB-5 (n = 12; Figure S4A). Colocalization increased and reached a maximum when most dorsal cell fusions have been completed (69%; 1.8-fold stage; n = 18; Figure S4B). At later stages, we found a gradual reduction in EFF-1 colocalization with RAB-5 (Figures S4C and S4D). In contrast, colocalization of EFF-1 with lysosomal LMP-1 increased from 8% at the 1.5-fold stage to 26% at the 2-fold stage when most epidermal fusions have been completed. To summarize, we observe only minor localization of EFF-1 at the apical and basolateral membranes; rather, most EFF-1 localizes to early endosomes, the endocytic pathway, and to the Golgi apparatus. After cells fuse, most of EFF-1 is localized to RAB-5-positive early endosomes and partially in lysosomes where EFF-1 may undergo degradation.
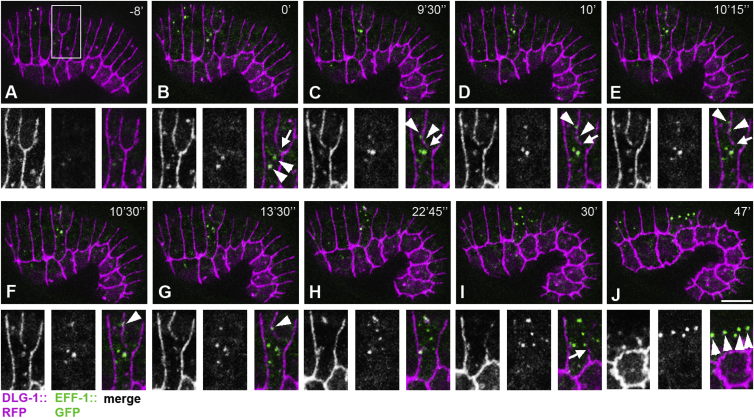
EFF-1 Shuttles to the Fusion Sites and Back to the Cell Interior within Vesicles
To determine whether wild-type EFF-1::GFP transiently shuttles to the plasma membrane and back to the intracellular early endosomes, we analyzed time-lapse movies. Followed by live imaging, EFF-1::GFP appeared dispersed in the cytoplasm within puncta in hypodermal cells ready to fuse (Figure 5B). Faint EFF-1::GFP puncta approached the cell junctions marked by DLG-1::RFP from both fusing cells transiently (Figures 5C–5E, arrowheads). About 10% of EFF-1::GFP puncta oscillated within 0.5 μm from the cell junction for 15 ± 6 min (n = 20 EFF-1::GFP puncta from eight embryos; Figures 5C–5G, arrowheads; Movies S1 and S2). During this time, 80% of the observed apical junctions began to disassemble (n = 20; Figures 5B–5E, arrows) to complete syncytia formation. The number of EFF-1::GFP-positive puncta increased during the process of cell fusion (Figures 5C–5I). After disassembly of the apical junctions, small EFF-1::GFP puncta merged into larger and brighter puncta (Figure 5J, arrowheads). Based on these findings, we hypothesize that EFF-1 is stored within early endosomes and is transiently transported to the cell surface when cells are ready to fuse.
Figure 5.
The Dynamics of EFF-1::GFP during Cell Fusion
Live imaging of EFF-1::GFP (green) and apical junction marker DLG-1::RFP (magenta) in the process of dorsal fusions. Fusing cells are highlighted in (A), and higher magnification inset is shown below in separate channels: DLG-1::RFP (left); EFF-1::GFP (middle); and merged image (right panel). (B–E) Early stages of apical junction disassembly (arrows). Dynamic colocalization of EFF-1 and DLG-1 on plasma membranes (arrowheads). Time points (in minutes and seconds) of image acquisition are presented in the upper right corners. Time 0’ indicates the beginning of Movie S1.
(A) EFF-1::GFP fluorescence is barely detectable in cells that are going to fuse.
(B) EFF-1::GFP appears in the cell cytoplasm within a pair of bright vesicles (arrowheads) and diffuse where the cell junction disassembles (arrow).
(C–E) EFF-1::GFP arrives at the cell junction from both fusing cells (C, arrowheads) and move along the junction (D and E, arrowheads). Arrows mark the edge of the cell junction undergoing disassembly.
(F) EFF-1 puncta coming from opposite cells join together on the cell junction (arrowhead).
(G and H) At the end of the first dorsal cell fusion, EFF-1::GFP puncta are distributed in the cytoplasm of the syncytium.
(I) Second junction discontinuity revealing the second cell fusion (arrow).
(J) EFF-1::GFP vesicles become larger, brighter, and aligned in an anterior-posterior line (arrowheads) within the intermediate syncytium. The scale bar represents 10 μm.
EFF-1 Is Dynamically Delivered to All Apical Domains, Including Ones that Do Not Fuse
It has previously been reported that EFF-1∗::GFP (∗, mutant protein; del Campo et al., 2005) accumulates only at the membranes between cells that are destined to fuse. However, the dynamic subcellular localization of EFF-1∗::GFP does not match our observations made by immunofluorescence staining using anti-EFF-1 monoclonal antibodies (Figures 1 and S5) and our EFF-1::GFP dynamic behavior (Figure 5; Movies S1 and S2). Furthermore, the EFF-1∗::GFP construct harbors two point mutations at highly conserved sites, T176A and N529D, and does not rescue eff-1(hy21) animals (Avinoam and Podbilewicz, 2011).
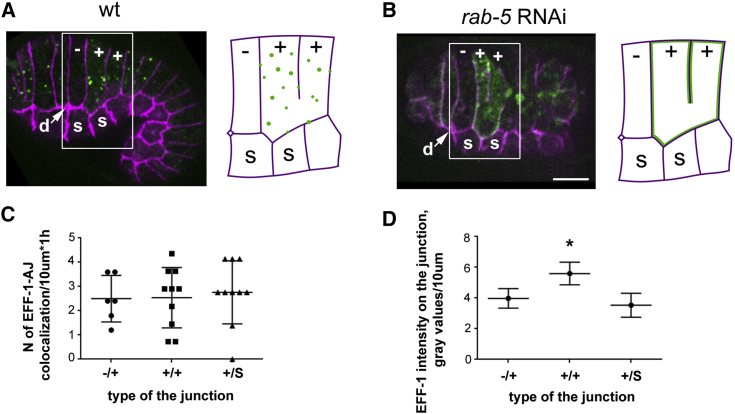
To test whether EFF-1 transport is specifically targeted to the plasma membrane domains where fusion occurs, we analyzed the directionality of EFF-1::GFP delivery to the apical junctions between the cells, which lie posterior to the deirid (d) (Figure 6A). In these examples, a dorsal hypodermal cell that is not expressing EFF-1::GFP at a given time point is marked by minus; the adjacent dorsal and posterior cells express EFF-1::GFP and are marked with plus; seam cells are marked with the letter “s”. We found that EFF-1::GFP is transiently localized to all apical plasma membrane domains in “+” cells (Figure 6A; Movie S5). There is no statistical difference between the number of EFF-1::GFP puncta transiently localized between EFF-1(+) and EFF-1(−), between two EFF-1s(+/+), and the junction between EFF-1/seam cells (+/s; Figure 6C). Thus, in contrast to the previous paradigm (del Campo et al., 2005), we found that EFF-1 is continuously and dynamically localizing to all apical plasma membrane domains and not only to those destined to fuse.
Figure 6.
EFF-1 Is Constitutively Transported to All Apical Plasma Membrane Domains
(A) EFF-1::GFP (green) puncta colocalization with the apical junctions marked DLG-1::RFP (magenta). On the picture and on the schematics, EFF-1-expressing cells are marked with “+,” epidermal cell that does not express EFF-1 is marked by “−“; d, deirid; s, seam cell. EFF-1::GFP is transiently localized to the junctions between different cell types: −/+; +/+; and +/s. Schematics represent highlighted cells from the picture; EFF-1::GFP (green puncta) transiently colocalizes with apical junctions (see Movie S2).
(B) After rab-5(RNAi), EFF-1::GFP is spread to all the junctions of −/+, +/+, and +/s types. In schematics, accumulation of EFF-1::GFP is visualized by the green line on the magenta apical junctions (see Movie S6).
(C) Categorical scatterplot of EFF-1::GFP vesicle colocalization to the different junctions per 10 μm of junction length per hour, measured in wild-type embryos. Each point represents number of events (colocalization) per junction over time. n = 8 embryos with a total of 6–10 junctions analyzed over ∼50 min. Middle line reflects the mean; upper and lower lines show SD. Thus, EFF-1-containing vesicles transiently associate (e.g., fuse) to all types of junctions. There is no statistical difference between data sets (Student’s t test).
(D) EFF-1::GFP relative intensity measured on the cell junctions in embryos treated with rab-5(RNAi). Five junctions of each type over ten time points were analyzed (n = 50); movies of three independent embryos were used (normalized to EFF-1::GFP gray values × 103/10 μm). Data are presented as mean (middle line) ± SD (upper and lower lines). There is no difference between intensities measured on −/+ and on +/s junction. ∗p < 0.05 (Student’s t test).
If EFF-1 is constitutively and nonspecifically internalized via receptor-mediated endocytosis from all plasma membrane domains, then following RAB-5(RNAi), EFF-1::GFP may be mislocalized to all apical domains and not only to the junctions destined to fuse. We found that EFF-1 transport to the plasma membrane was not affected whereas EFF-1 endocytosis was blocked by RAB-5(RNAi). As a result, EFF-1 was localized at all the apical plasma membrane domains of the + cells (Figure 6B; Movie S6). We then measured the intensities of EFF-1::GFP at the junctions between −/+ and +/s junctions. We found that the relative EFF-1::GFP intensities on the −/+ and +/s junctions are not statistically different (Figure 6D). For the membranes between two EFF-1-expressing cells (+/+), the intensity of EFF-1::GFP was higher, but this is to be expected as both cells contribute EFF-1 to this junction (Figure 6D). Thus, we show that EFF-1 transport to and from the plasma membrane has no specificity according to the junction type and the place of fusion. Based on our results, we propose that vesicles containing EFF-1::GFP cargo have the same probability of fusing with all apical plasma membranes.
In summary, EFF-1 localization in C. elegans embryonic epidermal cells is tightly maintained in early endosomes by the RAB-5- and DYN-1-dependent endocytic machinery. The EFF-1 protein is dynamically delivered to all apical plasma membranes transiently and without specificity to the place of fusion.
Discussion
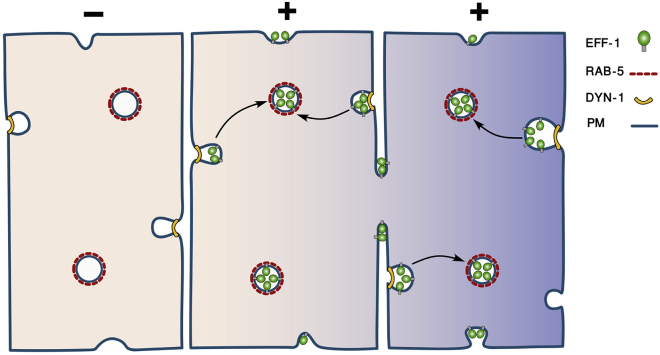
Based on our results, we propose a model for the regulation of EFF-1 localization and fusion activity by endocytosis. Synthesized EFF-1 transiently localizes to the surrounding apical plasma membranes of EFF-1-expressing cells. Membrane fusion is activated when the concentration of EFF-1 on two opposing membranes exceed a certain threshold and is mediated by homotypic interactions between EFF-1 proteins expressed from two cells (Podbilewicz et al., 2006). After constitutive non-selective targeting to all the apical domains of the plasma membrane, EFF-1 is recycled to early endosomes via dynamin/RAB-5-mediated trafficking (Figure 7). EFF-1 accumulates in RAB-5-positive endosomes probably because the rate of internalization is faster than the rate of transport to the apical plasma membranes. If EFF-1 trafficking is disrupted by RAB-5 knockdown or by DYN-1 downregulation, EFF-1 accumulates in the plasma membranes and can cause excessive cell fusion that contributes to embryonic lethality during elongation (morphogenesis).
Figure 7.
Model of Cell Fusion in C. elegans Embryonic Hypodermis
EFF-1 (green) is expressed in all surrounding plasma membranes (blue line) without relation to the future fusion-fated membranes. Cell that does not express EFF-1 (−) is colored in light khaki (left cell); fusion-fated cells that express EFF-1 (+) are colored in gradient of purple to khaki (central and right cells). The excess EFF-1 is actively removed from the cell surface via endocytosis in a dynamin- (yellow line) and RAB-5- (red dashed line) dependent mechanism. EFF-1 accumulates in the cytoplasm within early endosomes (circles outlined with the red dashed line). Only when EFF-1 is simultaneously present in both neighboring membranes, cell fusion is activated.
Traffic Defects May Cause EFF-1 Membrane Accumulation Prior to Fusion
Our results demonstrate that cell-cell fusion in C. elegans embryos requires transient and low level of EFF-1 localization to the fusing cell membranes (Figures S5A–S5D). In contrast to the results reported here, EFF-1 stable expression at the plasma membrane was observed in previous studies (Avinoam et al., 2011, del Campo et al., 2005). Indeed, an EFF-1∗::GFP was concentrated at cell-cell apical junctions in C. elegans embryos (Figure S5E, arrow; del Campo et al., 2005). However, we found that this EFF-1∗::GFP reporter carries two point mutations (T176A and N529D) and does not rescue eff-1(hy21) animals (Avinoam and Podbilewicz, 2011). We suggest that the mutations cause EFF-1∗::GFP abnormal accumulation in some apical junctions. Additionally, EFF-1 ectopically expressed under a heat shock promoter was detected at the plasma membranes of intestinal cells just before fusion in C. elegans embryos (Figure S5F, arrows). This was also true for EFF-1 that was ectopically expressed in nematode neurons, cultured insect, and mammalian cells (Figure S5G, arrows; Avinoam et al., 2011, Neumann et al., 2015, Podbilewicz et al., 2006, Zeev-Ben-Mordehai et al., 2014). These ectopic overexpression setups give rise to aberrant distribution of EFF-1 that does not mirror that of the highly regulated endogenous protein in the embryonic epidermis.
EFF-1 Fuses Cells Locally
According to the homotypic fusion model supported by recent biochemical and structural evidence, membrane fusion is mediated by EFF-1 trans-trimerization (Pérez-Vargas et al., 2014). The bright EFF-1 intracellular vesicles detected by immunofluorescence are likely to be composed of EFF-1 in a postfusion, trimeric conformation. It is unlikely that the different anti-EFF-1 antibodies we have used are trimer specific, because the same pattern was observed by EFF-1::GFP expression. Moreover, our monoclonal antibodies equally recognize both monomers and trimers by ELISA and western blot, including in samples of purified monomeric and trimeric EFF-1 ectodomains (data not shown). EFF-1::GFP vesicles became visible only at the onset of membrane fusion (within minutes before junction disassembly). We suggest that the concentration of EFF-1 monomers that is sufficient to initiate membrane fusion in vivo is low and barely detectable by confocal and superresolution microscopy. Localized high concentrations of EFF-1 monomers may be sufficient to initiate trans-EFF-1 complex formation and fusion.
EFF-1 vesicles are concentrated along lines that run parallel to the seam cells and are enriched with microtubules (Figure S1). We also observed that junction disassembly is often initiated at specific locations within cell junctions. These findings are compatible with the idea that microtubules mediate EFF-1 transport to specific places on the cell membranes from both cells undergoing fusion. EFF-1 local enrichment on both sides of the plasma membranes is sufficient to initiate cell fusion, as supported by genetic mosaic analyses of EFF-1-mediated fusion in C. elegans, in cell culture, and between viruses and cells (Avinoam et al., 2011, Podbilewicz et al., 2006).
EFF-1 Trafficking Is Regulated by RAB-5
Endocytosis plays an essential role in intercellular signaling, uptake of nutrients, and membrane recycling (Grant and Donaldson, 2009, McMahon and Boucrot, 2011, Mellman, 1996). RAB-5 is a central regulator of the early endocytic pathway and is a marker for the early endosome (Mizuno-Yamasaki et al., 2012, Sato et al., 2014, Zerial and McBride, 2001). Based on live imaging and immunolocalization, we propose that EFF-1 is transiently localized to the plasma membrane, internalized, and transported to early endosomes (Figure 7). There are two possibilities for how EFF-1 is transported from the plasma membrane to the early endosome. Some cells completely internalize their plasma membrane within 0.5–2 hr (Steinman et al., 1976), and EFF-1 can be endocytosed with the general membrane turnover. Another option is that EFF-1 transport is specific and mediated by an endocytosis signal (Traub, 2009). Trans-oligomerization of EFF-1 from opposing plasma membranes and the following conformational changes of trimers are proposed to dock the membranes and to initiate membrane fusion (Pérez-Vargas et al., 2014). Soluble DIII was shown to block EFF-1-mediated cell fusion in transfected mammalian cells, supporting the model based on class II viral fusion proteins in which DIII translocation from a linear pre-fusion conformation to a parallel postfusion hairpin conformation is required for membrane fusion (Pérez-Vargas et al., 2014). The localization of EFF-1::GFP in rab-5(RNAi) embryos shows mislocalization to all the apical plasma membranes including the domains that do not normally fuse (Figures 2B and 6B). Thus, EFF-1 is transported to all apical plasma membranes domains and not only to fusion-fated domains of the apical plasma membrane.
Is Endocytosis a Universal Regulator of Cell Fusion?
Because EFF-1 is a powerful fusogen, specialized safety mechanisms are required to prevent ectopic cell fusion. First, EFF-1 expression is regulated transcriptionally (Alper and Kenyon, 2002, Brabin et al., 2011, Cassata et al., 2005, Fernandes and Sternberg, 2007, Margalit et al., 2007, Mason et al., 2008, Pellegrino et al., 2011, Rasmussen et al., 2008, Shemer and Podbilewicz, 2002, Walser et al., 2006, Weinstein and Mendoza, 2013, Yi and Sommer, 2007). Gene-expression-based regulation may be the primary mechanism of specificity in EFF-1-mediated fusion. Second, EFF-1 expressed in one cell needs a partner from a neighboring cell in order to mediate fusion (Podbilewicz et al., 2006). Third, local concentration of EFF-1 on the plasma membrane is downregulated by dynamin/RAB-5-mediated endocytosis. Trafficking of EFF-1 may provide a fine-tuning to its fusion activity. During mammalian myoblast and osteoclast fusion, the opposite control mechanism was found to occur: cells required endocytosis and dynamin activity in order to fuse (Leikina et al., 2013, Shin et al., 2014, Verma et al., 2014). It is conceivable that endocytosis and recycling act during diverse cell-cell fusion events. Recently, the engulfment pathway was shown to act upstream of EFF-1 activity during regenerative axonal fusion in C. elegans (Neumann et al., 2015). In addition, gamete fusion in the mouse was linked to endocytosis and exocytosis (Satouh et al., 2012, Wassarman and Litscher, 2008). In Drosophila myoblast fusion, the adhesion molecule SNS, which is essential for fusion, was shown to colocalize with Rab-5 (Haralalka et al., 2014). Here, we found a clear case in which endocytosis negatively regulates EFF-1-mediated cell-cell fusion to prevent excessive syncytia formation, which can result in abnormalities and contributes to late embryonic lethality.
In conclusion, we found that the GTPases RAB-5 and dynamin control EFF-1 transient localization on the surface of cells destined to fuse and prevent excess fusion by dynamically and constitutively internalizing this fusion protein from all the apical domains of the plasma membrane.
Experimental Procedures
Strains and Transgenic Animals
All nematode strains were maintained according to standard protocols (Brenner, 1974). The list of C. elegans strains used in this study is in Tables S1 and S2 and Supplemental Experimental Procedures. For the construction of eff-1::gfp rescue strain, germline transformation was performed using standard protocols (Mello and Fire, 1995). eff-1::gfp fosmid was ordered from Transgenome (Sarov et al., 2012) and was injected into BP953 (eff-1(hy21)II; mcIs46[dlg-1::RFP]; Diogon et al., 2007) at 50 ng/μl concentration. Transgenic lines were kept as extrachromosomal arrays and maintained by following the rescue phenotype of eff-1(hy21ts) at 25°C.
Immunofluorescence
Embryos were prepared for immunostaining as described in Supplemental Experimental Procedures. The following primary antibodies were used at the dilutions indicated: α-EFF-1 (mouse ascites 20.10 and 10.5; 1:1,000); MH27 (α-AJM-1; mouse; 1:500); α-GFP (rabbit; 1:500; MBL); and α-tubulin (mouse; Sigma; 1:500). α-DLG-1 antibody (rabbit; 1:400) was a kind gift from Prof. Dr. Olaf Bossinger (RWTH Aachen University). MH46 (α-myotactin; mouse; 1:400; Hresko et al., 1994) was a kind gift from Dr. Limor Broday (Tel Aviv University); mouse monoclonal antibodies against C. elegans proteins DYN-1, CYP33E1, PAS-7, and HSP60 were obtained from Developmental Studies Hybridoma Bank and used at 1:10 dilution. Texas Red-X phalloidin (Molecular Probes) at a final concentration of 0.2 μM was added with the secondary antibody.
Anti-EFF-1 Antibody Production
Anti-EFF-1 antibodies were prepared against purified EFF-1EC obtained as described (Pérez-Vargas et al., 2014), and the hybridomas were prepared and screened by ELISA in the lab of Africa Gonzalez (Vigo University). 20.10 and 10.5 ascites were used for immunostaining of C. elegans embryos and larvae (Fridman, 2012).
Microscopy and Live Imaging
Images of fixed samples were taken on Zeiss LSM 700 confocal microscope with X63/1.4 PlanApo objective. Superresolution images were obtained using Elyra S.1 structured illumination system (SIM) with X63/1.4 PlanApo objective and EMCCD iXon camera (Andor). Confocal or SIM z stacks were analyzed and processed by Zen software (Zeiss).
For the imaging of live embryos, gravid adult hermaphrodites were dissected in 50 μl of 0.7× egg salts and embryos were moved to 2% agar pad with a mouth pipette. The specimen was covered with a coverslip (1.5H) and sealed with scotch tape to prevent drying. Images were acquired using spinning disc Revolution XD confocal system (Andor) based on Nikon Eclipse Ti microscope equipped with 100×/1.3 PlanFluor objective, perfect focus system; CSU-X1 spinning disc (Yokogawa) and iXon3 EMCCD camera (Andor) operated by IQ2 software (Andor). Image sequences were further processed and analyzed with ImageJ (NIH). Figures were prepared using Adobe Photoshop CS5 and Adobe Illustrator CS11.
Quantitation of Colocalization and Statistics
Colocalization was quantified manually on individual z slices (∼100 per embryo), analyzing red and green channels separately on superresolution SIM images using Zen software (Zeiss). Colocalization analysis of EFF-1 puncta was performed on embryos carrying the GFP-tagged protein of interest labeled with antibodies against GFP (Alexa 488; green channel) and with monoclonal antibodies against EFF-1 (Alexa Fluor 568; red channel). EFF-1 puncta were considered colocalized with puncta containing GFP marker if the areas of those puncta showed more than 50% overlay. The percentage of colocalization is expressed as the ratio of puncta positive for EFF-1 and GFP out of the total number of EFF-1 puncta in the embryonic hypodermis. For each marker, at least five embryos were quantified; data are shown as mean ± SEM (see Table S1). Significant differences between two groups of measurements were determined by the two-tailed unpaired t test using Excel. For statistical comparison of multiple groups, we used ANOVA test in Excel.
RNAi
RNAi by feeding was performed with an ORF-RNAi library (Rual et al., 2004). RNAi feeding protocol was used (Beifuss and Gumienny, 2012). We used dyn-1, rab-5, rabx-5, and rme-6 RNAi, bli-4 was used as a positive control and C06C3.5 as a negative control. For each clone, 5 ml LB media containing 50 μg/ml carbenicillin (Sigma) was inoculated with a single colony and incubated overnight at 37°C at 220 rpm. dsRNA production was induced by adding 1 mM IPTG (Sigma) to the cultures and additional incubation for 4 hr. Each bacterial culture (50 μl) was inoculated into NGM RNAi plate containing 50 μg/ml carbenicillin and 1 mM IPTG and dried. C. elegans L4 stage was isolated to NGM plates for 1 hr to get rid of OP50 bacteria in the intestine. Embryos were examined on the next day after larvae were transferred to plates with bacteria producing the specific dsRNAs.
Author Contributions
K.S. performed the experiments. B.P. did initial SIM experiments. K.S. and B.P. conceived the project, analyzed the data, and wrote the manuscript.
Acknowledgments
We thank B. Grant, O. Bossinger, M. Labouesse, B. Mohler, Z. Hong, A. Chisholm, and L. Broday for C. elegans strains. We acknowledge CGC (NIH Office of Research Infrastructure Programs P40 OD010440) and C. elegans knockout consortium for strains. We thank TransgenOme for eff-1p::eff-1::gfp fosmid; A. Gonzalez, O. Bossinger, L. Broday, and Developmental Studies Hybridoma Bank (U. of Iowa) for antibodies; C. Valansi for initial characterization of antibodies against EFF-1; O. Avinoam, D. Cassel, M. Hilliard, M. Oren-Suissa, and E. Schejter for critically reading the manuscript; and T. Rapoport and members of his lab for discussions. B.P. was a Grass fellow at Radcliffe Institute for Advanced Study at Harvard. K.S. was supported by the Ministry of Absorption, Israel (N061486). The work was funded by European Research Council (ERC) advanced grant 268843, GIF German-Israeli Foundation for Scientific Research and Development (grant 937/2006), US-Israel Binational Science Foundation (grant 2013151), and the Israel Science Foundation grant 443/12.
Published: February 4, 2016
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, five figures, three tables, and six movies and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2016.01.027.
Supplemental Information
Time lapse recording of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] transgenic embryo. EFF-1::GFP is shown in green, DLG-1::RFP is displayed in magenta. The z-series were recorded every 15 sec using spinning disk confocal microscopy, multiple intensity projection of a z-stack is shown at each time point. Lower panel represents enlarged area of the embryo (same embryo as in Figure 5). Arrows mark the start of apical junction disassembly. Time in minutes:seconds is shown at the top right corner.
Another embryo of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] strain showing later stages of hypodermis fusion. Arrows indicate the beginning of apical junction disassembly. Microscopy and time interval as in Movie S1.
Time lapse recording of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] embryo after rab-5(RNAi) treatment. Green represents EFF-1::GFP, magenta shows DLG-1::RFP. The z-series were recorded every 30 seconds, lower panel represents enlarged area of the fusion (Figure 2E). Arrow marks the beginning of apical junction disassembly. Note the disappearance of the bright EFF-1::GFP puncta, the localization of EFF-1::GFP on the junctions and the increase of numerous small and less bright EFF-1::GFP vesicular staining.
Animated z-stack of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] embryo treated with rab-5 RNAi. All cells in the dorsal hypodermis are fused to each other (hyperfusion) in contrast to three unfused syncytia (hyp5, 6, and 7) in the wt embryos (Figure 1A). EFF-1 is expressed on plasma membrane of hypodermis syncytia. Maximum intensity projection of the dorsal side of this embryo is shown in Supplemental Figure S2G, S2H.
Animated z-stack of live embryo expressing EFF-1::GFP and DLG-1::RFP after dyn-1 RNAi treatment. This embryo shows EFF-1::GFP expression on the apical membrane, defects in embryogenesis, and hyperfusion.
Time lapse recording of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] embryo after rab-5(RNAi) treatment. Green represents EFF-1::GFP, magenta shows DLG-1::RFP. The z-series were recorded every 30 seconds.
References
- Aguilar P.S., Baylies M.K., Fleissner A., Helming L., Inoue N., Podbilewicz B., Wang H., Wong M. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013;29:427–437. doi: 10.1016/j.tig.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper S., Kenyon C. The zinc finger protein REF-2 functions with the Hox genes to inhibit cell fusion in the ventral epidermis of C. elegans. Development. 2002;129:3335–3348. doi: 10.1242/dev.129.14.3335. [DOI] [PubMed] [Google Scholar]
- Avinoam O., Podbilewicz B. Eukaryotic cell-cell fusion families. Curr. Top. Membr. 2011;68:209–234. doi: 10.1016/B978-0-12-385891-7.00009-X. [DOI] [PubMed] [Google Scholar]
- Avinoam O., Fridman K., Valansi C., Abutbul I., Zeev-Ben-Mordehai T., Maurer U.E., Sapir A., Danino D., Grünewald K., White J.M., Podbilewicz B. Conserved eukaryotic fusogens can fuse viral envelopes to cells. Science. 2011;332:589–592. doi: 10.1126/science.1202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beifuss K.K., Gumienny T.L. RNAi screening to identify postembryonic phenotypes in C. elegans. J. Vis. Exp. 2012:e3442. doi: 10.3791/3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabin C., Appleford P.J., Woollard A. The Caenorhabditis elegans GATA factor ELT-1 works through the cell proliferation regulator BRO-1 and the Fusogen EFF-1 to maintain the seam stem-like fate. PLoS Genet. 2011;7:e1002200. doi: 10.1371/journal.pgen.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassata G., Shemer G., Morandi P., Donhauser R., Podbilewicz B., Baumeister R. ceh-16/engrailed patterns the embryonic epidermis of Caenorhabditis elegans. Development. 2005;132:739–749. doi: 10.1242/dev.01638. [DOI] [PubMed] [Google Scholar]
- Chen C.C., Schweinsberg P.J., Vashist S., Mareiniss D.P., Lambie E.J., Grant B.D. RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol. Biol. Cell. 2006;17:1286–1297. doi: 10.1091/mbc.E05-08-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E.H., Grote E., Mohler W., Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Clark S.G., Shurland D.L., Meyerowitz E.M., Bargmann C.I., van der Bliek A.M. A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans. Proc. Natl. Acad. Sci. USA. 1997;94:10438–10443. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo J.J., Opoku-Serebuoh E., Isaacson A.B., Scranton V.L., Tucker M., Han M., Mohler W.A. Fusogenic activity of EFF-1 is regulated via dynamic localization in fusing somatic cells of C. elegans. Curr. Biol. 2005;15:413–423. doi: 10.1016/j.cub.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Diogon M., Wissler F., Quintin S., Nagamatsu Y., Sookhareea S., Landmann F., Hutter H., Vitale N., Labouesse M. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development. 2007;134:2469–2479. doi: 10.1242/dev.005074. [DOI] [PubMed] [Google Scholar]
- Fares H., Greenwald I. Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics. 2001;159:133–145. doi: 10.1093/genetics/159.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J.S., Sternberg P.W. The tailless ortholog nhr-67 regulates patterning of gene expression and morphogenesis in the C. elegans vulva. PLoS Genet. 2007;3:e69. doi: 10.1371/journal.pgen.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman, K. (2012). Ultrastructure and function of AFF-1 and EFF-1 in membrane remodeling. MSc thesis (Haifa: Technion).
- Gattegno T., Mittal A., Valansi C., Nguyen K.C., Hall D.H., Chernomordik L.V., Podbilewicz B. Genetic control of fusion pore expansion in the epidermis of Caenorhabditis elegans. Mol. Biol. Cell. 2007;18:1153–1166. doi: 10.1091/mbc.E06-09-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B., Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B.D., Donaldson J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralalka S., Shelton C., Cartwright H.N., Guo F., Trimble R., Kumar R.P., Abmayr S.M. Live imaging provides new insights on dynamic F-actin filopodia and differential endocytosis during myoblast fusion in Drosophila. PLoS ONE. 2014;9:e114126. doi: 10.1371/journal.pone.0114126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko M.C., Williams B.D., Waterston R.H. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J. Cell Biol. 1994;124:491–506. doi: 10.1083/jcb.124.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Ren Y., Ng W.P., Li S., Son S., Kee Y.S., Zhang S., Zhang G., Fletcher D.A., Robinson D.N., Chen E.H. Mechanical tension drives cell membrane fusion. Dev. Cell. 2015;32:561–573. doi: 10.1016/j.devcel.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontani K., Moskowitz I.P., Rothman J.H. Repression of cell-cell fusion by components of the C. elegans vacuolar ATPase complex. Dev. Cell. 2005;8:787–794. doi: 10.1016/j.devcel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Kozlov M.M., Campelo F., Liska N., Chernomordik L.V., Marrink S.J., McMahon H.T. Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 2014;29:53–60. doi: 10.1016/j.ceb.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E., Melikov K., Sanyal S., Verma S.K., Eun B., Gebert C., Pfeifer K., Lizunov V.A., Kozlov M.M., Chernomordik L.V. Extracellular annexins and dynamin are important for sequential steps in myoblast fusion. J. Cell Biol. 2013;200:109–123. doi: 10.1083/jcb.201207012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois S., Benedetto A., Garnier J.M., Schwab Y., Labouesse M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J. Cell Biol. 2006;173:949–961. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick V.P., Fox D.T., Spradling A.C. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr. Biol. 2013;23:2224–2232. doi: 10.1016/j.cub.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V., Zhang L., Viloria-Petit A.M., Ogunjimi A.A., Inanlou M.R., Chiu E., Buchanan M., Hosein A.N., Basik M., Wrana J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Margalit A., Neufeld E., Feinstein N., Wilson K.L., Podbilewicz B., Gruenbaum Y. Barrier to autointegration factor blocks premature cell fusion and maintains adult muscle integrity in C. elegans. J. Cell Biol. 2007;178:661–673. doi: 10.1083/jcb.200704049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D.A., Rabinowitz J.S., Portman D.S. dmd-3, a doublesex-related gene regulated by tra-1, governs sex-specific morphogenesis in C. elegans. Development. 2008;135:2373–2382. doi: 10.1242/dev.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H.T., Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Medvinsky A., Smith A. Stem cells: Fusion brings down barriers. Nature. 2003;422:823–825. doi: 10.1038/422823a. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mellman I., Yarden Y. Endocytosis and cancer. Cold Spring Harb. Perspect. Biol. 2013;5:a016949. doi: 10.1101/cshperspect.a016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C., Fire A. DNA transformation. In: Epstein H.F., Shakes D.C., editors. Methods in Cell Biology Caenorhabditis elegans: Model Biological Analysis of an Organism. Academic Press; San Diego: 1995. pp. 451–482. [Google Scholar]
- Messa M., Fernández-Busnadiego R., Sun E.W., Chen H., Czapla H., Wrasman K., Wu Y., Ko G., Ross T., Wendland B., De Camilli P. Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits. eLife. 2014;3:e03311. doi: 10.7554/eLife.03311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E., Rivera-Molina F., Novick P. GTPase networks in membrane traffic. Annu. Rev. Biochem. 2012;81:637–659. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler W.A., Shemer G., del Campo J.J., Valansi C., Opoku-Serebuoh E., Scranton V., Assaf N., White J.G., Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev. Cell. 2002;2:355–362. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Neumann B., Coakley S., Giordano-Santini R., Linton C., Lee E.S., Nakagawa A., Xue D., Hilliard M.A. EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature. 2015;517:219–222. doi: 10.1038/nature14102. [DOI] [PubMed] [Google Scholar]
- Nishi T., Forgac M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- Oren-Suissa M., Podbilewicz B. Evolution of programmed cell fusion: common mechanisms and distinct functions. Dev. Dyn. 2010;239:1515–1528. doi: 10.1002/dvdy.22284. [DOI] [PubMed] [Google Scholar]
- Pellegrino M.W., Farooqui S., Fröhli E., Rehrauer H., Kaeser-Pebernard S., Müller F., Gasser R.B., Hajnal A. LIN-39 and the EGFR/RAS/MAPK pathway regulate C. elegans vulval morphogenesis via the VAB-23 zinc finger protein. Development. 2011;138:4649–4660. doi: 10.1242/dev.071951. [DOI] [PubMed] [Google Scholar]
- Pérez-Vargas J., Krey T., Valansi C., Avinoam O., Haouz A., Jamin M., Raveh-Barak H., Podbilewicz B., Rey F.A. Structural basis of eukaryotic cell-cell fusion. Cell. 2014;157:407–419. doi: 10.1016/j.cell.2014.02.020. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B. Virus and cell fusion mechanisms. Annu. Rev. Cell Dev. Biol. 2014;30:111–139. doi: 10.1146/annurev-cellbio-101512-122422. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B., White J.G. Cell fusions in the developing epithelial of C. elegans. Dev. Biol. 1994;161:408–424. doi: 10.1006/dbio.1994.1041. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B., Leikina E., Sapir A., Valansi C., Suissa M., Shemer G., Chernomordik L.V. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev. Cell. 2006;11:471–481. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Rasmussen J.P., English K., Tenlen J.R., Priess J.R. Notch signaling and morphogenesis of single-cell tubes in the C. elegans digestive tract. Dev. Cell. 2008;14:559–569. doi: 10.1016/j.devcel.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi A.Z., Swain J.R., Davies P.S., Bailey A.S., Decker A.D., Willenbring H., Grompe M., Fleming W.H., Wong M.H. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:6321–6325. doi: 10.1073/pnas.0508593103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual J.F., Ceron J., Koreth J., Hao T., Nicot A.S., Hirozane-Kishikawa T., Vandenhaute J., Orkin S.H., Hill D.E., van den Heuvel S. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M., Murray J.I., Schanze K., Pozniakovski A., Niu W., Angermann K., Hasse S., Rupprecht M., Vinis E., Tinney M. A genome-scale resource for in vivo tag-based protein function exploration in C. elegans. Cell. 2012;150:855–866. doi: 10.1016/j.cell.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Sato K. Dynamic regulation of autophagy and endocytosis for cell remodeling during early development. Traffic. 2013;14:479–486. doi: 10.1111/tra.12050. [DOI] [PubMed] [Google Scholar]
- Sato K., Norris A., Sato M., Grant B.D. C. elegans as a model for membrane traffic. WormBook. 2014:1–47. doi: 10.1895/wormbook.1.77.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satouh Y., Inoue N., Ikawa M., Okabe M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 2012;125:4985–4990. doi: 10.1242/jcs.100867. [DOI] [PubMed] [Google Scholar]
- Schmid S.L., Sorkin A., Zerial M. Endocytosis: Past, present, and future. Cold Spring Harb. Perspect. Biol. 2014;6:a022509. doi: 10.1101/cshperspect.a022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer G., Podbilewicz B. LIN-39/Hox triggers cell division and represses EFF-1/fusogen-dependent vulval cell fusion. Genes Dev. 2002;16:3136–3141. doi: 10.1101/gad.251202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer G., Suissa M., Kolotuev I., Nguyen K.C., Hall D.H., Podbilewicz B. EFF-1 is sufficient to initiate and execute tissue-specific cell fusion in C. elegans. Curr. Biol. 2004;14:1587–1591. doi: 10.1016/j.cub.2004.07.059. [DOI] [PubMed] [Google Scholar]
- Shilagardi K., Li S., Luo F., Marikar F., Duan R., Jin P., Kim J.H., Murnen K., Chen E.H. Actin-propelled invasive membrane protrusions promote fusogenic protein engagement during cell-cell fusion. Science. 2013;340:359–363. doi: 10.1126/science.1234781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N.Y., Choi H., Neff L., Wu Y., Saito H., Ferguson S.M., De Camilli P., Baron R. Dynamin and endocytosis are required for the fusion of osteoclasts and myoblasts. J. Cell Biol. 2014;207:73–89. doi: 10.1083/jcb.201401137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn-Thomas J.H., Mohler W.A. New insights into the mechanisms and roles of cell-cell fusion. Int. Rev. Cell Mol. Biol. 2011;289:149–209. doi: 10.1016/B978-0-12-386039-2.00005-5. [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Brodie S.E., Cohn Z.A. Membrane flow during pinocytosis. A stereologic analysis. J. Cell Biol. 1976;68:665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L.M. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- Verma S.K., Leikina E., Melikov K., Chernomordik L.V. Late stages of the synchronized macrophage fusion in osteoclast formation depend on dynamin. Biochem. J. 2014;464:293–300. doi: 10.1042/BJ20141233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser C.B., Battu G., Hoier E.F., Hajnal A. Distinct roles of the Pumilio and FBF translational repressors during C. elegans vulval development. Development. 2006;133:3461–3471. doi: 10.1242/dev.02496. [DOI] [PubMed] [Google Scholar]
- Wassarman P.M., Litscher E.S. Mammalian fertilization is dependent on multiple membrane fusion events. Methods Mol. Biol. 2008;475:99–113. doi: 10.1007/978-1-59745-250-2_6. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Liu Q., Davis M.W., Hollopeter G., Thomas N., Jorgensen N.B., Jorgensen E.M. Ultrafast endocytosis at Caenorhabditis elegans neuromuscular junctions. eLife. 2013;2:e00723. doi: 10.7554/eLife.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein N., Mendoza L. A network model for the specification of vulval precursor cells and cell fusion control in Caenorhabditis elegans. Front. Genet. 2013;4:112. doi: 10.3389/fgene.2013.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi B., Sommer R.J. The pax-3 gene is involved in vulva formation in Pristionchus pacificus and is a target of the Hox gene lin-39. Development. 2007;134:3111–3119. doi: 10.1242/dev.008375. [DOI] [PubMed] [Google Scholar]
- Zeev-Ben-Mordehai T., Vasishtan D., Siebert C.A., Grünewald K. The full-length cell-cell fusogen EFF-1 is monomeric and upright on the membrane. Nat. Commun. 2014;5:3912. doi: 10.1038/ncomms4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer A., Gilleron J., Bogorad R.L., Marsico G., Nonaka H., Seifert S., Epstein-Barash H., Kuchimanchi S., Peng C.G., Ruda V.M. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485:465–470. doi: 10.1038/nature11133. [DOI] [PubMed] [Google Scholar]
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time lapse recording of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] transgenic embryo. EFF-1::GFP is shown in green, DLG-1::RFP is displayed in magenta. The z-series were recorded every 15 sec using spinning disk confocal microscopy, multiple intensity projection of a z-stack is shown at each time point. Lower panel represents enlarged area of the embryo (same embryo as in Figure 5). Arrows mark the start of apical junction disassembly. Time in minutes:seconds is shown at the top right corner.
Another embryo of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] strain showing later stages of hypodermis fusion. Arrows indicate the beginning of apical junction disassembly. Microscopy and time interval as in Movie S1.
Time lapse recording of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] embryo after rab-5(RNAi) treatment. Green represents EFF-1::GFP, magenta shows DLG-1::RFP. The z-series were recorded every 30 seconds, lower panel represents enlarged area of the fusion (Figure 2E). Arrow marks the beginning of apical junction disassembly. Note the disappearance of the bright EFF-1::GFP puncta, the localization of EFF-1::GFP on the junctions and the increase of numerous small and less bright EFF-1::GFP vesicular staining.
Animated z-stack of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] embryo treated with rab-5 RNAi. All cells in the dorsal hypodermis are fused to each other (hyperfusion) in contrast to three unfused syncytia (hyp5, 6, and 7) in the wt embryos (Figure 1A). EFF-1 is expressed on plasma membrane of hypodermis syncytia. Maximum intensity projection of the dorsal side of this embryo is shown in Supplemental Figure S2G, S2H.
Animated z-stack of live embryo expressing EFF-1::GFP and DLG-1::RFP after dyn-1 RNAi treatment. This embryo shows EFF-1::GFP expression on the apical membrane, defects in embryogenesis, and hyperfusion.
Time lapse recording of an eff-1(hy21)II; mcIs46[dlg-1::RFP]; hyEx160[peff-1::eff-1::GFP] embryo after rab-5(RNAi) treatment. Green represents EFF-1::GFP, magenta shows DLG-1::RFP. The z-series were recorded every 30 seconds.