Abstract
Aims
The transcatheter mitral valve interventions (TRAMI) registry was established in order to assess safety and efficacy of catheter-based mitral valve interventional techniques in Germany, and prospectively enrolled 828 MitraClip patients (median age 76 years, median log. EuroSCORE I 20.0%) between August 2010 and July 2013. We present the 1-year outcome in this MitraClip cohort—which is the largest published to date.
Methods and results
Seven forty-nine patients (90.5%) were available for 1-year follow-up and included in the following analyses. Mortality, major adverse cardiovascular event rates, and New York Heart Association (NYHA) classes were recorded. Predictors of 1-year mortality were identified by multivariate analysis using a Cox regression model with stepwise forward selection. The 1-year mortality was 20.3%. At 1 year, 63.3% of TRAMI patients pertained to NYHA functional classes I or II (compared with 11.0% at baseline), and self-rated health status (on EuroQuol visual analogue scale) also improved significantly by 10 points. Importantly, a significant proportion of patients regained the complete independence in self-care after MitraClip implantation (independence in 74.0 vs. 58.6% at baseline, P = 0.005). Predictors of 1-year mortality were NYHA class IV (hazard ratio, HR 1.62, P = 0.02), anaemia (HR 2.44, P = 0.02), previous aortic valve intervention (HR 2.12, P = 0.002), serum creatinine ≥1.5 mg/dL (HR 1.77, P = 0.002), peripheral artery disease (HR 2.12, P = 0.0003), left ventricular ejection fraction <30% (HR 1.58, P = 0.01), severe tricuspid regurgitation (HR 1.84, P = 0.003), and procedural failure (defined as operator-reported failure, conversion to surgery, failure of clip placement, or residual post-procedural severe mitral regurgitation) (HR 4.36, P < 0.0001).
Conclusions
Treatment of significant MR with MitraClip resulted in significant clinical improvements in a high proportion of TRAMI patients after 12 months. In the TRAMI cohort, the failure of procedural success exhibited the highest hazard ratio concerning the prediction of 1-year mortality.
Keywords: MitraClip, Percutaneous mitral valve repair, Mitral valve regurgitation, Heart failure, TRAMI registry
Introduction
Mitral regurgitation (MR), the most common type of valvular heart disease, affects nearly 10% of people above the age of 75 years.1 A recent European analysis2 demonstrated that ∼50% of patients with severe symptomatic MR were denied surgical mitral valve interventions (mostly due to advanced age, impaired left ventricular function and a high burden of comorbidities) indicating the need for less invasive treatment alternatives.
The percutaneous edge-to-edge mitral valve repair with MitraClip (Abbott, Menlo Park, CA, USA) is based on the surgical technique first described by Alfieri.3 Feasibility in a porcine model4 and the first human case5 were both published in 2003. In 2009, the EVEREST I trial (Endovascular Valve Edge-to-Edge Repair Study),6 demonstrated safety and feasibility of MitraClip implantation for treatment of MR. Subsequently, EVEREST II,7 a multicentre randomized controlled trial, compared percutaneous repair vs. surgery in operable patients with symptomatic severe MR (≥3+). The percutaneous repair was associated with superior safety and similar improvements in clinical outcomes. However, patients treated percutaneously more commonly required additional surgical procedures for treatment of residual MR at 12 months (20 vs. 2.2%, P < 0.001). At 4 years of follow-up of EVEREST II patients,8 there was no significant difference in mortality (17 vs. 18%, P = 0.9) and incidence of MR ≥3+ (22 vs. 25%, P = 0.745) between the two groups. Surgery for significant residual MR occurred in 25% of percutaneously treated patients vs. 5.5% of surgically treated patients indicating that only few surgeries were required after the first year of follow-up.
Whereas EVEREST II enrolled only operable patients with predominantly primary MR, large registries on MitraClip therapy demonstrated that real-world patients differ significantly from the EVEREST II cohort9–11 underlining the continuous need for outcome data derived from industry-independent multicentre real-world studies.
In Germany, catheter-based mitral valve repair has rapidly been accepted at many centres and is performed at increasing numbers. To date, the independent German transcatheter mitral valve interventions (TRAMI) registry comprises the largest multicentre cohort of patients treated with MitraClip implantation world-wide. In the following, we present complete 1-year outcome data of the prospective TRAMI section and aim at identifying predictors of 1-year mortality.
Methods
Transcatheter mitral valve interventions registry
The non-randomized TRAMI registry (also named German mitral valve registry) was established in 2010 in order to assess safety and efficacy of catheter-based mitral valve interventional techniques (both for stenosis and regurgitation) and was made available to all sites in Germany performing such therapies. The vast majority of patients enrolled in TRAMI underwent MitraClip® implantation. Detailed descriptions of the registry and initial results have recently been published.9,12–17 The registry was organized into a prospective and a retrospective section. Prospective patient enrolment began in August 2010 and ended in July 2013. Follow-up for the prospective section is performed centrally by the ‘Institut für Herzinfarktforschung (IHF)’ at the Heart Center Ludwigshafen at 30 days and at 1, 3 and 5 years. One-year follow-up data were collected until July 2014 by standardized telephone interview. Participating centres were also encouraged to enter retrospectively all patients treated with MitraClip between January 2009 and July 2010 and perform follow-up visits according to institutional practice. These patients were not included in the study due to lack of standardized follow-up and lower data quality. The following analyses rely exclusively on patients who were prospectively enrolled into TRAMI and who were available for 1-year follow-up. All patients gave written informed consent. Data were collected via web-based electronic case report forms by the IHF Ludwigshafen. Importantly, the TRAMI registry is independent from industry. The majority of funding was provided by proprietary means of the IHF and additional funding by ‘Deutsche Herzstiftung e.V.’
Assessment of mitral regurgitation, device, and procedure
The severity of MR was graded in three grades as I (mild), II (moderate), and III (severe) based on current recommendations18 and was evaluated at each individual centre. The MitraClip system, a polyester-covered cobalt-chromium V-shaped device with two movable arms, received CE Mark in 2008. The implantation procedure was performed as previously described.6,7,19
Definitions
In the TRAMI registry, procedural failure was defined as severe residual MR, abortion of MitraClip procedure, conversion to open heart surgery, or failure as assessed by the interventional team: In addition to the criteria mentioned first, the operator could classify an intervention as unsuccessful due to subjective reasons (i.e. non-significant MR reduction). Major adverse cardiac and cerebrovascular events (MACCE) included death from any cause, stroke, and myocardial infarction.
Quality of life
For evaluation of health-related quality of life, the EQ-5D-3L,20,21 one of the most common generic questionnaires was used. It essentially comprises two sections. The EQ-5D descriptive system consists of five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) each of which can take one of three responses recording three levels of severity (no problems/some or moderate problems/extreme problems). The EQ visual analogue scale (EQ VAS) records the respondent's self-rated health on a vertical scale where the endpoints are labelled ‘Best imaginable health state, 100’ and ‘Worst imaginable health state, 0’. This information can be used as a quantitative measure of health outcome as judged by the individual respondents.
Statistical analysis
Categorical variables are presented by absolute numbers and percentages and are compared by χ2 test. Continuous variables are expressed as means with standard deviations or medians with interquartile ranges and are compared by Mann–Whitney–Wilcoxon test. The cumulative one-year incidence of mortality and MACCE was estimated by the Kaplan–Meier method.
Multivariable Cox regression using stepwise forward selection was performed to analyse the influence of relevant variables on 1-year mortality. We included all variables correlated with 1-year mortality at P < 0.1 or expected to influence outcome from previous publications. To avoid collinearity, the surgical risk scores (log. EuroSCORE and STS Score) were excluded, because some of their components were inserted into the model.
The change within the EuroQoL five dimensions and EQ-5D score between baseline and 1-year FU was tested using the Sign test.
All tests were two tailed and P-values <0.05 were considered significant. SAS statistical package version 9.3 (Cary, NC, USA) was used for the computations.
Results
Baseline characteristics
Between August 2010 and July 2013, 828 patients were enrolled prospectively into the TRAMI registry in 21 German sites (a complete list of participating sites is provided in Supplementary material online, Table S1). One-year follow-up could be performed in 749 patients (90.5%) at a median of 386 days after MitraClip implantation. Only patients with available 1-year follow-up were considered in the following analyses (with the exception of the Cox regression model). Concerning the missing individuals, 16/79 withdrew their consent, and 63/79 were lost to follow-up.
Baseline characteristics of the 749 remaining participants are displayed in Table 1. Patients enrolled in TRAMI were predominantly male (61.4%) and characterized by advanced age (median, 76.0 years), high estimated surgical risk (median log EuroSCORE 20.0%, median STS score 6.0%), and a high burden of comorbidities (i.e. coronary artery disease 78.1%, renal failure 65.5%, severely reduced LVEF 33.7%). Furthermore, patients were in advanced stages of heart failure (NYHA class III in 70.5%, NYHA class IV in 18.5%). Consecutively, they exhibited reduced functional capacities (median 6 min walk test distance: 200.0 [IQR: 120.0–320.0] m) and had elevated levels of NT-pro BNP and BNP (median NT-pro BNP: 3497.0 [IQR: 1559.0–6880.5] pg/mL; median BNP: 692.0 [IQR: 244.0–1380.0] pg/mL). The prevalent valvular pathogenesis was secondary MR (71.3%).
Table 1.
Baseline characteristics of patients undergoing MitraClip implantation in different registries and trials
| EVEREST II (n = 184) | ACESS-EU (n = 567) | Transcatheter Valve Treatment Sentinel Pilot Registry (n = 628) | TRAMI (prospective cohort) (n = 749) | |
|---|---|---|---|---|
| Age (years) | 67.3 ± 12.8a | 73.7 ± 9.6 | 74.2 ± 9.7 | 76.0 [71.0–81.0]b |
| Female gender, n (%) | 69/184 (37.5%) | 205/567 (36.2%) | 232/628 (36.9%) | 289/749 (38.6%) |
| NYHA functional class III/IV, n (%) | 94/184 (51.1%) | 466/549 (84.9%) | 537/628 (85.5%) | 646/726 (89.0%) |
| Left ventricular ejection fraction | ||||
| LVEF (%) | 60.0 ± 10.1a | NA | 42.6 ± 15.9 | NA |
| LVEF, <30%, n (%) | NA | 193/562 (34.3%) | 206/628 (32.8%) | 236/700 (33.7%) |
| LVEF, 30–50%, n (%) | NA | NA | NA | 247/700 (35.3%) |
| LVEF >50%, n (%) | NA | NA | NA | 217/700 (31.0%) |
| Aetiology of MR, n (%) | ||||
| Secondary | 49/184 (26.6%) | 393/510 (77.1%) | 452/628 (72.0%) | 478/670 (71.3%)c |
| Primary | 135/184 (73.4%) | 117/510 (22.9%) | 176/628 (28.0%) | 172/618 (27.8%)c |
| Severity of MR, n (%) | ||||
| 3+/4+ (1+, 2+, 3+, 4+) | 176/184 (95.7%) | 554/567 (97.7%) | NA | NA |
| Severe (mild, moderate, severe) | NA | NA | 541/368 (86.1%) | 660/704 (93.8%) |
| Comorbidities, n (%) | ||||
| Coronary artery disease | 86/183 (47.0%) | 354/565 (62.7%) | 194/628 (30.9%) | 424/543 (78.1%) |
| Previous myocardial infarction | 40/183 (21.9%) | 175/547 (32.0%) | 196/628 (31.2%) | 201/721 (27.9%) |
| Previous stroke | NA | 73/566 (12.9%) | 90/628 (14.4%) | 76/718 (10.6%) |
| Atrial fibrillation | 59/175 (33.7%) | 356/526 (67.7%) | 199/628 (31.7%) | 319/724 (44.1%) |
| Diabetes mellitus | 14/184 (7.6%) | 168/567 (29.6%) | 175/628 (27.9%) | 226/719 (31.4%) |
| COPD | 27/183 (14.8%) | 107/562 (19.0%) | 121/628 (19.3%) | 160/718 (22.3%) |
| Renal failure | 6/184 (3.3%) | 236/567 (41.6%) | 192/628 (30.5%) | 468/714 (65.5%) |
| Previous CABG | 38/184 (20.7%) | 164/567 (28.9%) | 203/628 (32.3%) | 186/724 (25.7%) |
| Previous AVR or TAVR | NA | NA | NA | 66/724 (9.1%) |
| Previous MV surgery or intervention | 0 | NA | NA | 14/726 (1.9%) |
| Previous valve surgery | NA | NA | 65/628 (10.4%) | NA |
| Estimated surgical risk | ||||
| Log. EuroSCORE (%) | NA | 23.0 ± 18.3 | 20.4 ± 16.7 | 20.0 [12.0–31.0] |
| STS score (%) | 5.0 ± 4.0 | NA | NA | 6.0 [4.0–11.0] |
aMean ± SD.
bMedian, IQR.
cSum not 100% because of indeterminate aetiology.
Compared with our study cohort, the 79 patients who were excluded due to missing 1-year follow-up exhibited a slightly higher estimated surgical risk at baseline documented by significantly higher values for log. EuroSCORE (median, 22.5 vs. 20%, P = 0.03) and systolic pulmonary artery pressure (P < 0.01), and by a tendency towards a higher age (P = 0.06) and a higher prevalence of COPD (P = 0.07) (see Supplementary material online, TableSSupplementary Data).
Patient allocation
The decision for patient allocation to percutaneous therapy was left to the discretion of the participating centres and was made by a heart team in 60.9%, by a cardiologist alone in 37.8%, and by a cardiac surgeon in only 1.3% of patients. The most common reason for denying surgery was estimated surgical high-risk status (58.0% of cases, log. EuroSCORE ≥20% in 50% of patients), followed by age (48.3%), patient's preference (25.0%), frailty (21.2%), limited prognosis due to non-cardiac (mostly malignant) comorbidity (22.3%), and inoperability (11.4%) (entry of more than one reason was possible).
In-hospital and 30-day outcome
In TRAMI, MitraClip implantation was successful in most cases. On average, procedural time was 102.8 ± 54.1 min, radiation time 28.8 ± 57.9 min, and 1.4 ± 0.6 clips were implanted. Procedural failure (defined as severe residual MR, abortion of MitraClip procedure, conversion to open heart surgery, or failure as assessed by the interventional team) was observed in only 3.2% of patients. Also, the intervention proved to be safe: Intra-procedural death occurred in only one patient (0.1%), in-hospital mortality was 2.4% (n = 18), and MACCE rate was 3.1% (n = 6 cases of stroke, n = 0 of myocardial infarction).
Specific procedural complications are displayed in Table 2. The most common adverse events were respiratory failure with consecutive invasive or non-invasive ventilation (6.5%), and severe bleeding necessitating transfusion (7.0%). No case of clip embolization occurred, but single-leaflet clip attachment was observed in five cases (0.7%). Consecutively, an additional mitral valve procedure became necessary during the index hospitalization in 11 patients (surgery in six and percutaneous interventions in five cases). The median length of hospital stay was 9 days [IQR 6.0–15.0]. The vast majority of patients (89.3%) were regularly discharged to their normal social environment, 6.3% to cardiac rehabilitation facilities, and the remaining to nursing homes. Echocardiography at time of discharge revealed the presence of severe MR in only 2.3% of cases, whereas 85.2% had none or mild MR. At 30 days, exact cumulative mortality was 4.5%.
Table 2.
In-hospital/30 days outcomes after MitraClip implantation in different registries and trials
| EVEREST II (n = 184) | ACESS-EU (n = 567) | Transcatheter Valve Treatment Sentinel Pilot Registry (n = 628) | TRAMI (prospective cohort) (n = 749) | |
|---|---|---|---|---|
| Hospital stay (days) | NA | 7.7 ± 8.2 (median: 6.0) | 5.0 [3.0–7.0] | 9.0 [6.0–15.0] |
| Procedural success | ||||
| (Clip implanted + MR ≤2+/not severe) | 137/178 (77.0%) | 516/567 (91.0%) | 599/628 (95.4%) | 719/741 (97.0%) |
| Mitral regurgitation at discharge, n (%) | ||||
| None/mild | NA | NA | 268/368 (72.8%) | 631/741 (85.2%) |
| Moderate | NA | NA | 93/368 (25.3%) | 93/741 (12.6%) |
| Severe | NA | NA | 7/368 (1.9%) | 17/741 (2.3%) |
| 0–1+ | NA | 50.9% | NA | NA |
| 2+ | NA | 40.3% | NA | NA |
| 3+/4+ | 41/178 (23%) | 8.8% | NA | NA |
| Adverse events | All events until day 30 | All events until day 30 | In-hospital events | In-hospital events |
| MACCE (death, MI, and stroke) | NA | NA | NA | 22/712 (3.1%) |
| In-hospital mortality | NA | 11/563 (2.0%) | 18/628 (2.9%) | 18/749 (2.4%) |
| 30-day mortality | 2/184 (1%) | 19/567 (3.4%) | NA | 34/749 (4.5%) |
| Myocardial infarction | 0/184 (0%) | 4/567 (0.7%) | 0/628 (0%) | 0/711 (0.0%) |
| Stroke | 2/184 (1%) | 4/567 (0.7%) | 1/628 (0.2%) | 6/712 (0.8%) |
| Non-MACCE | ||||
| TIA | NA | NA | NA | 6/712 (0.8%) |
| Respirat. failure (re-intubation) | NA | 4/567 (0.7%) | NA | 16/711 (2.3%) |
| Severe bleeding, transfusion | 24/184 (13%) | 22/567 (3.9%) | 70/628 (11.2%) | 50/711 (7.0%) |
| Low cardiac output | NA | NA | NA | 9/710 (1.3%) |
| Pericardial tamponade | 3/184 (1.6%) | 6/567 (1.1%) | 7/628 (1.1%) | 12/710 (1.7%) |
| Clip embolization | 0/184 (0%) | 0/567 (0%) | 4/628 (0.7%) | 0/710 (0.0%) |
| Partial clip detachment | 9/184 (4.9%) | 27/567 (4.8%) | NA | 5/749 (0.7%) |
| Additional MV procedure, n (%) | 28/184 (15.2%) | 16/567 (2.8%) | NA | 11/710 (1.5%) |
| Surgical | 28/184 (15.2%) | 6/567 (1.1%) | NA | 6/710 (0.8%) |
| Percutaneous | 0 | 10/567 (1.7%) | NA | 5/710 (0.7%) |
Compared with our study cohort, the 79 patients who were excluded due to missing 1-year follow-up had a significantly lower rate of procedural success (92.2 vs. 96.8%, P = 0.03) and consecutively a higher rate of additional mitral valve procedures at 30 days (P = 0.02, see Supplementary material online, TableSSupplementary Data). However, in-hospital mortality in this group was (per definition) 0%, and the incidence of all other complications did not differ.
One-year. outcomes: safety, efficacy, heart failure, and quality of life
Adverse events and measures of treatment efficacy at 12 months are listed in Table 3. Exact cumulative mortality at 1 year was 20.3% (152/749 patients) (see also Figure 1). Causes of death were the following: sudden unexpected death in 23/152 cases (15.1%), other cardiovascular causes in 56/152 patients (36.8%), non-cardiovascular reasons in 19/152 cases (12.5%), and unknown/ unreported reasons in 54/152 patients (35.5%). The cumulative incidences of TIA, stroke and myocardial infarction at 1 year were 3.8%, 2.1% and 0.9%, respectively. During the first year of follow-up, 14.1% of patients were re-hospitalized due to cardiac decompensation and 17.8% due to other cardiovascular reasons. An additional mitral valve procedure became necessary in cumulatively 8.5% of patients (surgery in 2.3% and second MitraClip implantation in 5.2%).
Table 3.
One-year outcomes after MitraClip implantation in different registries and trials
| EVEREST II (n = 184) | ACESS-EU (n = 567) | Transcatheter Valve Treatment Sentinel Pilot Registry European Sentinel Pilot Registry (n = 552/628) | TRAMI (prospective cohort) (n = 749) | |
|---|---|---|---|---|
| Mitral regurgitation at 1 year, n (%) | ||||
| None/mild | NA | NA | 216/368 (58.6%) | NA |
| Moderate | NA | NA | 130/368 (35.4%) | NA |
| Severe | NA | NA | 22/368 (6.0%) | NA |
| 0+/1+ | 84/153 (54.9%) | 100/327 (30.6%) | NA | NA |
| 2+ | 41/153 (26.8%) | 158/327 (48.3%) | NA | NA |
| 3+/4+ | 28/153 (18.3%) | 69/327 (21.1%) | NA | NA |
| NYHA class at 1 year, n (%) | ||||
| I–II | 180/184 (97.8%) | 245/343 (71.4%) | 265/357 (74.2%) | 305/482 (63.3%) |
| III–IV | 4/184 (2.2%) | 98/343 (28.6%) | 92/357 (25.8%) | 177/482 (36.7%) |
| Quality of life | ||||
| EQ-5D-3L (compared with baseline) | NA | NA | NA | |
| Mobility | NA | NA | NA | 0.58 |
| Self-care | NA | NA | NA | <0.001 |
| Usual activities | NA | NA | NA | 0.89 |
| Pain/discomfort | NA | NA | NA | 0.21 |
| Anxiety/depression | NA | NA | NA | <0.0001 |
| EQ VAS (compared with baseline) | NA | NA | NA | 60.0 [50–70] vs. 50.0 [40–60]; P < 0.0001 |
| 36-item short-form Health Survey (compared with baseline) | Improvement (P < 0.001) | NA | NA | NA |
| MLHFQ (compared with baseline) | NA | Improvement of 13.5 ± 20.5 points (P < 0.0001) | NA | NA |
| 6MWT (compared with baseline) | NA | Improvement of 59.5 ± 112.4 m (P < 0.0001) | NA | NA |
| Adverse events | ||||
| MACCE (death, MI, and stroke) | ||||
| Death | 11/181 (6.1%) | 98/567 (17.3%) | 15.3% (Kaplan–Meier curve) | 152/749 (20.3%) |
| Myocardial infarction | 1/184 (0.5%) | 8/567 (1.4%) | NA | 4/425 (0.9%) |
| Stroke | 2/184 (1.1%) | 6/567 (1.1%) | NA | 9/423 (2.1%) |
| Non-MACCE | ||||
| TIA | 1/184 (0.5%) | NA | NA | 16/426 (3.8%) |
| Bleeding complications | 5/184 (2.7%) | 27/567 (4.8%) | NA | 56/443 (12.6%) |
| Need for resuscitation | 2/184 (1.1%) | 12/567 (2.1%) | NA | 9/426 (2.1%) |
| Rehospitalizations, n (%) | NA | NA | NA | 364/566 (64.3%) |
| Cardiac decompensation | NA | NA | 22.8% (Kaplan–Meier curve) | 80/566 (14.1%) |
| Other cardiac reason | NA | NA | NA | 101/566 (17.8%) |
| Non-cardiac reason | NA | NA | NA | 146/566 (25.8%) |
| Additional MV procedure, n (%) | 37/181 (21%) | 55/567 (9.7%) | 17/444 (3.8%) | 37/436 (8.5%) |
| Surgical | 37/181 (21%) | 36/ 567 (6.3%) | 4/444 (0.9%) | 10/436 (2.3%) |
| Percutaneous | 0/181 (0%) | 19/567 (3.4%) | 13/444 (2.9%) | 23/436 (5.2%) |
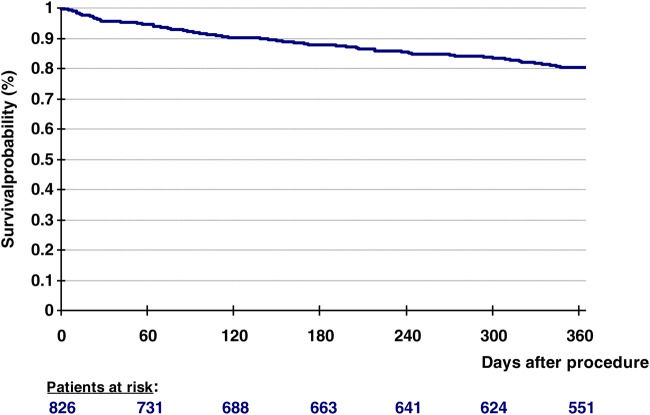
Figure 1.
Kaplan–Meier curve displaying overall survival after MitraClip implantation in transcatheter mitral valve interventions registry patients.
At 1 year, 63.3% of patients had no or few symptoms of heart failure and pertained to NYHA functional classes I or II (in contrast to 11.0% at baseline). Health-related quality of life was measured by the EQ-5 dimensions descriptive system and by the EQ VAS. Compared with baseline, significantly more patients were completely independent concerning the dimension ‘self-care’ (74.0 vs. 58.6%, P = 0.005), and significantly more patients reported no problems concerning the dimension ‘anxiety/depression’ (66.7 vs. 48.9%, P < 0.0001). Regarding the dimensions ‘mobility’, ‘usual activities,’ and ‘pain/discomfort’, no significant changes could be observed. However, patients' self-rated health status on the EQ VAS improved significantly from 50.0 [IQR 40.0–60.0] at baseline to 60.0 [IQR 50.0–70.0] at 1 year (P < 0.0001).
Predictors of 1-year mortality
In order to identify risk factors for long-term mortality (cumulative hospital and post-hospital mortality), baseline and procedural characteristics were compared between survivors and non-survivors at 1 year. Effects that proved to be statistically significant in univariable analysis were further subjected to multivariable Cox regression analysis, as well as the covariates ‘gender’ and ‘age>75 years'. Due to high numbers of missing values, the covariates ‘total procedure time’, ‘fluoroscopy time,’ and ‘PAP sys >45 mmHg’ were not used for the Cox regression model. The discharge medication was omitted in order to keep the 18 cases of in-hospital mortality for calculation. Due to redundancies, the surgical risk scores (which were predictive for mortality in univariable analysis) were not inserted into the model. Finally, the covariates ‘sinusrhythm, prior stroke, ‘number of implanted clips ≥2’, ‘COPD’, and ‘prior cardiac decompensation’ were excluded by the forward selection procedure (Table 4).
Table 4.
Predictors of 1-year mortality in the transcatheter mitral valve interventions registry cohort
| Multivariable analysis (Cox regression model) |
||
|---|---|---|
| HR (95% CI) | P | |
| Age >75 years | 1.29 (0.90–1.87) | 0.16 |
| Female gender | 1.13 (0.78–1.64) | 0.53 |
| NYHA IV | 1.62 (1.10–2.40) | 0.02 |
| Anaemia | 2.44 (1.16–5.12) | 0.02 |
| Previous aortic valve intervention | 2.12(1.32–3.41) | 0.002 |
| Creatinine ≥1.5 mg/dL | 1.77 (1.24–2.54) | 0.002 |
| Peripheral artery disease | 2.12 (1.41–3.20) | 0.0003 |
| LVEF <30% | 1.58 (1.10–2.31) | 0.01 |
| Severe tricuspid regurgitation | 1.84 (1.23–2.77) | 0.003 |
| Procedural failurea | 4.36 (2.37–8.02) | <0.0001 |
aOperator-reported failure, conversion to surgery, abortion of procedure or severe residual mitral regurgitation.
According to our multivariable analysis, significant predictors of 1-year mortality were NYHA class IV (HR 1.62, P = 0.02), anaemia (HR 2.44, P = 0.02), previous aortic valve intervention (HR 2.12, P = 0.002), serum creatinine ≥ 1.5 mg/dL (HR 1.77, P = 0.002), peripheral artery disease (HR 2.12, P = 0.0003), left ventricular ejection fraction <30% (HR 1.59, P = 0.01), severe tricuspid regurgitation (HR 1.84, P = 0.003), and procedural failure (HR 4.36, P < 0.0001).
In order to test the prognostic performance of the developed risk model, we calculated the area (AUC) under the receiver operating characteristic curve: The quality of the regression model could be confirmed by a high c-value (0.75). In addition, we internally validated the model by using a five-fold cross-validation. Therefore, the study population was randomly divided into five equal groups. For each run, the AUC was calculated in the training group and then applied to the test group. Combining all the five test groups, we obtained an AUC average of 0.685, indicating the validity of the model.
Discussion
To date, the industry-independent TRAMI registry comprises the largest real-world cohort of patients treated by percutaneous edge-to-edge mitral valve repair with MitraClip. In the following, we will contrast the early and 1-year data of 749 TRAMI patients with the initial EVEREST II-cohort7 on the one hand, and with the 2 largest series of MitraClip patients published so far on the other hand: The ACCESS-EU study, a prospective European multicenter non-randomized post-approval study, enrolled 567 patients in 14 centres in Germany, Italy and Denmark. Early and 1-year results were published in 2013.10 Subsequently, the independent Transcatheter Valve Treatment Sentinel Pilot Registry (TCVT registry) reported immediate and 1-year results of 628 patients treated with MitraClip in 25 centres in 8 European countries in 2014.11
Baseline characteristics
The baseline demographics of the entire prospective TRAMI cohort are very similar to those that have already been published as preliminary results.9 In concordance with other registries,10,11 the TRAMI data also underline that patients treated with MitraClip implantation in contemporary ‘real life’ are very different from the initial EVEREST cohort (for comparison of baseline characteristics, see Table 1). In current European practice, MitraClip patients are older (in TRAMI ∼10 years older than in EVEREST II), in more advanced stages of heart failure (NYHA class III/IV: 89% in TRAMI, 86% in TCVT, 85% in ACCESS-EU, and 51% in EVEREST II), have the opposite distribution of MR aetiology (secondary MR in 71% of cases in TRAMI, 72% in TCVT, and 77% in ACCESS-EU, vs. in 27% of cases in EVEREST II), and exhibit a higher burden of comorbidities (see Table 1).7,10,11 Comparing the three registries, a tendency towards the treatment of increasingly older and sicker patients seems to be present, with patients at highest age, highest stages of heart failure and highest prevalence of specific comorbidities like coronary artery disease and renal failure enrolled in the TRAMI registry. Importantly, subgroup analyses in EVEREST II7 had identified patients with an age of at least 70 years and patients with functional MR as subgroups in which surgery was not superior to percutaneous treatment with regard to efficacy—cohorts, which are indeed treated with MitraClip implantation today according to TRAMI data. Thus, the low-risk percutaneous MitraClip implantation is in fact (and as intended according to current guidelines)18 reserved for patients who are no surgical candidates (due to high age, high estimated surgical risk, multiple comorbidities, and predominantly secondary aetiology of MR) in contemporary clinical practice in Germany, whereas operable patients with primary MR are still treated by conventional surgery according to current guidelines.18
In-hospital outcome
Despite patients' high-risk status, the incidence of specific procedural complications in TRAMI was comparable with previous reports (see Table 2), with stroke rates <1% and no peri-procedural myocardial infarctions. As in other publications, the most common adverse event was severe bleeding (7.0% in TRAMI); the incidence varied between 3.9% in ACCESS-EU10 and 13% in EVEREST II,7 most likely driven by strictness of definitions. In-hospital and 30 days mortality in TRAMI were 2.4 and 4.5% (30 days mortality according to Kaplan–Meier analysis: 4.3% [95%CI: 3.1–5.9%]),which was well in line with other registries (ACCESS-EU: 2.0%/3.4%; TCVT: 2.9%/not reported).
Median length of hospital stay after MitraClip implantation was longer in TRAMI than in previous reports (9 days in TRAMI, 6 days in ACCESS-EU,10 and 5 days in TCVT),11 probably reflecting national treatment practices and the high burden of comorbidity in the TRAMI cohort. However, the vast majority of patients could directly be discharged home suggesting a quick recovery from the procedure.
In TRAMI, the rate of procedural success was particularly high (97%). If compared with earlier reports (in which procedural success was commonly defined as MR reduction to ≤2+), an improvement of success rate in chronological order of publication (between 2009 and 2014) is noticeable: 74% in EVEREST I,19 77% in EVEREST II,7 80% in the EVEREST II High Risk Cohort,22 80% in the PERMIT-CARE study,23 85% in the Swiss registry,24 91% in ACCESS-EU,10 and 95% in TCVT),11 reflecting the growing experience with this complex technique over time. Accordingly, the need for additional mitral valve procedures during the index hospitalization occurred less frequently in TRAMI (1.3%) than in other reports (15.2% in EVEREST II,7 2.8% in ACCESS-EU,10 not reported for TCVT).
The echocardiographic results of the procedure cannot be directly compared due to different assessment of MR severity (Grades 0+, 1+, 2+, 3+, 4+ in EVEREST II and ACCESS-EU; grades none, mild, moderate, severe in TRAMI and TCVT). Furthermore, echo data were core-lab-adjudicated only in EVEREST II and ACCESS-EU, but not in TRAMI and TCVT. In ACCESS-EU, MR grade 0–1+ was present in 51% of patients, whereas 73% of TCVT patients and 76% of TRAMI patients had none or mild MR at time of discharge (site-reported).
1-year. outcomes: safety, efficacy, heart failure, and quality of life
One-year mortality in TRAMI (20.3%; according to Kaplan–Meier analysis 19.8% [95% CI: 17.2–22.8%]; see also Figure 1 and Table 3) was slightly higher than in ACCESS-EU (17.3%)10 and in TCVT (15.3%),11 but not unexpected high in a cohort at advanced age, with a high burden of comorbidity, and with nearly 60% of patients reporting heart failure hospitalizations during the 6 months before enrolment in TRAMI. For comparison: Jhund et al.25 examined long-term trends in survival after a first hospitalization for heart failure (1986–2003) and found a slightly improved but still poor prognosis even in 2003, with a 1-year mortality of 27.6% (95% CI: 24.5–31.1) in men and of 25.6% (95% CI: 22.6–28.8) in women. However, in the absence of a meaningful comparator group, a possible survival benefit cannot be estimated. This burning issue is addressed by ongoing clinical trials with randomization of MitraClip implantation against optimal medical therapy in patients with secondary MR.
The prevention of repeated hospital admissions in heart failure patients is a second valid target of medical interventions. Heart failure-related re-hospitalizations during the first year after MitraClip implantation occurred less frequently in TRAMI-patients (14.1%) than in the TCVT cohort (22.8%)11 (ACCESS-EU does not report the incidence of re-hospitalizations).
Moreover, the interventional success can be measured by the incidence of repeat mitral valve procedures. In TRAMI, an additional intervention became necessary in 8.5% of patients, whereas 9.7% in ACCESS-EU10 and 3.8% in TCVT11 underwent repeat procedures (in contrast to 21% in EVEREST II).7 Whereas the second interventions had been predominantly surgical in the earlier studies (21% in EVEREST II,7 6.3% in ACCESS-EU),10 mainly repeat MitraClip procedures were carried out in the younger registries (5.3% in TRAMI, 2.9% in TCVT).11 The decreasing indication for re-interventions in the more recent publications and the trend towards the performance of repeat clip implantations both mirror growing knowledge and technical experience in MitraClip interventions in European clinical practice.
In the absence of randomized trials in contemporary real-world cohorts, the consideration of functional outcomes is the most appropriate possibility to assess the efficacy of MitraClip therapy. At 1 year, 63.3% of TRAMI patients pertained to NYHA functional classes I or II (compared with 11.0% at baseline), and self-rated health status (on EQ VAS) also improved significantly by 10 points. Importantly, a significant proportion of patients regained the complete independence in self-care after MitraClip implantation (independence in 74.0 vs. 58.6% at baseline, P = 0.005), an issue of utmost importance in the context of health care economy. Given that most patients included in TRAMI had no surgical option, the expectation of clinical benefit from the procedure has been met in the majority of cases: The procedural success translated into improvements in heart failure class and quality-of-life-measures. Likewise, a significant NYHA functional class improvement, a significantly increased 6-min walking test distance, and a significantly improved result in the Minnesota Living with heart failure Quality of Life-Questionnaire could be documented in ACCESS-EU10 12 months after MitraClip therapy. The TCVT registry11 did not contain quality-of-life-measures, but in this registry the highest proportion of patients pertained to NYHA classes I and II after 1 year of follow-up (74.2%).
Furthermore, we aimed at identifying predictors of 1-year mortality in the TRAMI cohort. According to multivariable analysis, the procedural failure had the highest hazard ratio concerning the prediction of 1-year mortality (HR 4.36) (see also Table 4). Similarly, the failure of procedural success has also been identified as important predictor of mortality in a German single-centre cohort26 and in the Swiss registry.24 Conversely, a successful clip deployment was independently associated with the composite endpoint (death or heart failure-related readmission) at 1 year in TCVT patients (OR 0.12).11 Thus, the successful procedure with meaningful MR reduction seems to be the best guarantee of a favourable course. In patients at prohibitive surgical risk enrolled in the EVEREST II-trial,27 the degree of residual MR also was significantly associated with worse outcome. Patients who were discharged with MR severity of ≤1+ or of 2+ had comparable survival rates at 12 months (83.3 vs. 80.0%, P = 0.61). In contrast, 1-year survival was significantly reduced in patients with MR 3+/4+ at discharge—commonly referred to as procedural failures (52.4%, P = 0.001 in comparison with MR ≤1+ and P = 0.02 in comparison with MR 2+, respectively).
Other predictors of 1-year mortality in TRAMI were NYHA class IV (HR 1.62), anaemia (HR 2.44), previous aortic valve intervention (HR 2.12), renal failure with serum creatinine ≥1.5 mg/dL (HR 1.77), peripheral artery disease (HR 2.12), left ventricular ejection fraction <30% (HR 1.59), and severe tricuspid regurgitation (HR 1.84) (Table 4; the surgical risk scores were excluded from multivariable analysis due to redundancies). Regarding previous publications, EuroSCORE (OR 1.44) and LVEF <30% (OR 2.69) were identified as additional independent risk factors in the TCVT cohort.11
Limitations
First of all, the current data are reassuring that the results of percutaneous mitral valve repair with MitraClip seen in other registries are reproducible. Naturally, registries cannot replace randomized evidence and have the inherent limitation to be not completely controlled with the risk of under-reporting of events or complications. Although randomized trials are the foundation to establish evidence-based guidance in patient management, ‘all-comer’ registries like TRAMI serve an important complementary role by evaluating penetration and contemporary use of novel therapies in real life.
However, this study has several limitations. Because TRAMI was not industry-sponsored (which is also a strength of this registry), patient enrolment was on a voluntary basis, and no remuneration was paid. Therefore, the large range of patients included across centres probably reflects non-consecutive enrolment in several centres. All baseline and in-hospital data were site-reported. Moreover, echocardiographic data were not core-lab adjudicated and therefore of minor quality. Follow-up at 30 days and at 1 year was performed by telephone call and did therefore not include an echocardiography. Furthermore, regarding the entire cohort of 828 patients prospectively included in TRAMI, the 1-year follow-up was incomplete (90.5%) which could create a selection bias. To address this potential problem, Supplementary material online, Tables S2 and S3 comparing TRAMI patients with and without 1-year follow-up are presented.
Conclusions
Treatment of significant MR with MitraClip is efficacious and results in significant clinical improvements in a high proportion of TRAMI patients after 12 months. In the TRAMI cohort, the failure of procedural success exhibited the highest hazard ratio concerning the prediction of 1-year mortality. However, randomized studies to verify the efficacy of MitraClip therapy and prospective studies to define anatomical criteria that allow a better prediction of procedural success are still required.
Authors’ contributions
T.O.: performed statistical analysis. W.S.: handled funding and supervision and made critical revision of the manuscript for key intellectual content. M.P.: acquired the data, conceived and designed the research, drafted the manuscript.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The majority of funding of TRAMI is provided by proprietary means of the Stiftung Institut für Herzinfarktforschung (IHF)/Ludwigshafen. Additional funding is provided by Deutsche Herzstiftung e.V. Funding to pay the Open Access publication charges for this article was provided by IFS GmbH (Institut für anwendungsorientierte Forschung und klinische Studien, institute for clinical studies), Von-Bar-Str. 2/4, 37075 Göttingen.
Conflict of interest: R.S.v.B., W.S. and H.S. received personal fees, M.P. and W.S. received travel expenses and J.S. received a grant from Abbott Vascular Germany.
Supplementary Material
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Mirabel M, Iung B, Baron G, Messika-Zeitoun D, Detaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358–1365. [DOI] [PubMed] [Google Scholar]
- 3. Alfieri O, Maisano F, De BM, Stefano PL, Torracca L, Oppizzi M, La CG. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001;122:674–681. [DOI] [PubMed] [Google Scholar]
- 4. St Goar FG, Fann JI, Komtebedde J, Foster E, Oz MC, Fogarty TJ, Feldman T, Block PC. Endovascular edge-to-edge mitral valve repair: short-term results in a porcine model. Circulation 2003;108:1990–1993. [DOI] [PubMed] [Google Scholar]
- 5. Condado JA, Acquatella H, Rodriguez L, Whitlow P, Velez-Gimo M, St Goar FG. Percutaneous edge-to-edge mitral valve repair: 2-year follow-up in the first human case. Catheter Cardiovasc Interv 2006;67:323–325. [DOI] [PubMed] [Google Scholar]
- 6. Feldman T, Wasserman HS, Herrmann HC, Gray W, Block PC, Whitlow P, St GF, Rodriguez L, Silvestry F, Schwartz A, Sanborn TA, Condado JA, Foster E. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol 2005;46:2134–2140. [DOI] [PubMed] [Google Scholar]
- 7. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395–1406. [DOI] [PubMed] [Google Scholar]
- 8. Mauri L, Foster E, Glower DD, Apruzzese P, Massaro JM, Herrmann HC, Hermiller J, Gray W, Wang A, Pedersen WR, Bajwa T, Lasala J, Low R, Grayburn P, Feldman T. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013;62:317–328. [DOI] [PubMed] [Google Scholar]
- 9. Baldus S, Schillinger W, Franzen O, Bekeredjian R, Sievert H, Schofer J, Kuck KH, Konorza T, Mollmann H, Hehrlein C, Ouarrak T, Senges J, Meinertz T. MitraClip therapy in daily clinical practice: initial results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2012;14:1050–1055. [DOI] [PubMed] [Google Scholar]
- 10. Maisano F, Franzen O, Baldus S, Schafer U, Hausleiter J, Butter C, Ussia GP, Sievert H, Richardt G, Widder JD, Moccetti T, Schillinger W. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052–1061. [DOI] [PubMed] [Google Scholar]
- 11. Nickenig G, Estevez-Loureiro R, Franzen O, Tamburino C, Vanderheyden M, Luscher TF, Moat N, Price S, Dall'Ara G, Winter R, Corti R, Grasso C, Snow TM, Jeger R, Blankenberg S, Settergren M, Tiroch K, Balzer J, Petronio AS, Buttner HJ, Ettori F, Sievert H, Fiorino MG, Claeys M, Ussia GP, Baumgartner H, Scandura S, Alamgir F, Keshavarzi F, Colombo A, Maisano F, Ebelt H, Aruta P, Lubos E, Plicht B, Schueler R, Pighi M, Di MC. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011–2012 Pilot European Sentinel Registry. J Am Coll Cardiol 2014;64:875–884. [DOI] [PubMed] [Google Scholar]
- 12. Ledwoch J, Franke J, Baldus S, Schillinger W, Bekeredjian R, Boekstegers P, Hink U, Kuck KH, Ouarrak T, Mollmann H, Nickenig G, Senges J, Franzen O, Sievert H. Impact of the learning curve on outcome after transcatheter mitral valve repair: results from the German Mitral Valve Registry. Clin Res Cardiol 2014;103:930–937. [DOI] [PubMed] [Google Scholar]
- 13. Schillinger W, Hunlich M, Baldus S, Ouarrak T, Boekstegers P, Hink U, Butter C, Bekeredjian R, Plicht B, Sievert H, Schofer J, Senges J, Meinertz T, Hasenfuss G. Acute outcomes after MitraClip therapy in highly aged patients: results from the German TRAnscatheter Mitral valve Interventions (TRAMI) Registry. EuroIntervention 2013;9:84–90. [DOI] [PubMed] [Google Scholar]
- 14. Wiebe J, Franke J, Lubos E, Boekstegers P, Schillinger W, Ouarrak T, May AE, Eggebrecht H, Kuck KH, Baldus S, Senges J, Sievert H. Percutaneous mitral valve repair with the MitraClip system according to the predicted risk by the logistic EuroSCORE: preliminary results from the German Transcatheter Mitral Valve Interventions (TRAMI) Registry. Catheter Cardiovasc Interv 2014;84:591–598. [DOI] [PubMed] [Google Scholar]
- 15. Rudolph V, Huntgeburth M, von Bardeleben RS, Boekstegers P, Lubos E, Schillinger W, Ouarrak T, Eggebrecht H, Butter C, Plicht B, May A, Franzen O, Schofer J, Senges J, Baldus S. Clinical outcome of critically ill, not fully recompensated, patients undergoing MitraClip therapy. Eur J Heart Fail 2014;16:1223–1229. [DOI] [PubMed] [Google Scholar]
- 16. Eggebrecht H, Schelle S, Puls M, Plicht B, von Bardeleben RS, Butter C, May AE, Lubos E, Boekstegers P, Ouarrak T, Senges J, Schmermund A. Risk and outcomes of complications during and after MitraClip implantation: experience in 828 patients from the German TRAnscatheter mitral valve interventions (TRAMI) registry. Catheter Cardiovasc Interv 2015;86:728–735. [DOI] [PubMed] [Google Scholar]
- 17. Schillinger W, Senges J. [TRAMI (Transcatheter Mitral Valve Interventions) register. The German mitral register]. Herz 2013;38:453–459. [DOI] [PubMed] [Google Scholar]
- 18. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De BM, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 19. Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009;54:686–694. [DOI] [PubMed] [Google Scholar]
- 20. Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53–72. [DOI] [PubMed] [Google Scholar]
- 21. Brooks R. Quality of life measures. Crit Care Med 1996;24:1769. [DOI] [PubMed] [Google Scholar]
- 22. Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, Smalling R, Bajwa T, Herrmann HC, Lasala J, Maddux JT, Tuzcu M, Kapadia S, Trento A, Siegel RJ, Foster E, Glower D, Mauri L, Kar S. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 2012;59:130–139. [DOI] [PubMed] [Google Scholar]
- 23. Auricchio A, Schillinger W, Meyer S, Maisano F, Hoffmann R, Ussia GP, Pedrazzini GB, van der Heyden J, Fratini S, Klersy C, Komtebedde J, Franzen O. Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol 2011;58:2183–2189. [DOI] [PubMed] [Google Scholar]
- 24. Surder D, Pedrazzini G, Gaemperli O, Biaggi P, Felix C, Rufibach K, Maur CA, Jeger R, Buser P, Kaufmann BA, Moccetti M, Hurlimann D, Buhler I, Bettex D, Scherman J, Pasotti E, Faletra FF, Zuber M, Moccetti T, Luscher TF, Erne P, Grunenfelder J, Corti R. Predictors for efficacy of percutaneous mitral valve repair using the MitraClip system: the results of the MitraSwiss registry. Heart 2013;99:1034–1040. [DOI] [PubMed] [Google Scholar]
- 25. Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, Chalmers JW, Capewell S, McMurray JJ. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation 2009;119:515–523. [DOI] [PubMed] [Google Scholar]
- 26. Puls M, Tichelbacker T, Bleckmann A, Hunlich M, von der Ehe K, Beuthner BE, Ruter K, Beissbarth T, Seipelt R, Schondube F, Hasenfuss G, Schillinger W. Failure of acute procedural success predicts adverse outcome after percutaneous edge-to-edge mitral valve repair with MitraClip. EuroIntervention 2014;9:1407–1417. [DOI] [PubMed] [Google Scholar]
- 27. Lim DS, Reynolds MR, Feldman T, Kar S, Herrmann HC, Wang A, Whitlow PL, Gray WA, Grayburn P, Mack MJ, Glower DD. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation after transcatheter mitral valve repair. J Am Coll Cardiol 2014;64:182–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.