Abstract
Foxp3-expressing regulatory T cells (Tregs) reside in tissues where they control inflammation and mediate tissue-specific functions. The skin of mice and humans contain a large number of Tregs; however, the mechanisms of how these cells function in skin remain largely unknown. Here, we show that Tregs facilitate cutaneous wound healing. Highly activated Tregs accumulated in skin early after wounding and specific ablation of these cells resulted in delayed wound re-epithelialization and kinetics of wound closure. Tregs in wounded skin attenuated IFNγ production and pro-inflammatory macrophage accumulation. Upon wounding, Tregs induce expression of the epidermal growth factor receptor (EGFR). Lineage-specific deletion of EGFR in Tregs resulted in reduced Treg accumulation and activation in wounded skin, delayed wound closure and increased pro-inflammatory macrophage accumulation. Taken together, our results reveal a novel role for Tregs in facilitating skin wound repair and suggest that Tregs utilize the EGFR pathway to mediate these effects.
INTRODUCTION
Foxp3-expressing regulatory T (Tregs) cells play an indispensable role in establishing and maintaining immune homeostasis. Although previously thought to be a relatively homogenous population, it has become increasingly accepted that Tregs residing in peripheral tissues possess tissue-specific functions. Both mouse and human skin contain a large number of tissue-resident Tregs (1, 2). However, the molecular mechanisms utilized by Tregs in skin are largely unknown. In addition, it is currently unknown if Tregs in skin play important roles in tissue-specific functions.
The skin is highly susceptible to traumatic injury. As such, wound healing is an extremely common and vital process coordinately mediated by multiple cell types and molecular pathways. The epidermal growth factor receptor (EGFR) pathway plays a major role in skin wound healing through stimulating epidermal and dermal regeneration (3). Interestingly, this pathway has also been shown to play a role in immune cell function. The EGFR ligand, AREG, is expressed by Tregs, where it enhances muscle and lung tissue repair after injury (4, 5). In addition, EGFR has been shown to be expressed on Tregs, where it plays a role in augmenting their suppressive capacity in vitro (6).
Because Tregs play a major role in mediating skin immune homeostasis, we set out to determine if these cells play a role in attenuating wound-associated inflammation. In addition, we set out to determine whether Tregs in skin utilize the EGFR pathway to facilitate wound repair. We show that upon full thickness wounding, highly activated Tregs accumulate in skin and play a major role in limiting IFNγ production and pro-inflammatory macrophage accumulation. Specific ablation of Tregs early after wounding resulted in delayed wound re-epithelialization and kinetics of wound closure. Tregs in skin induce expression of EGFR early after wounding and utilized this pathway to attenuate wound-associated inflammation and facilitate normal wound repair.
MATERIALS AND METHODS
Mice
All animal studies were performed in compliance with institutional guidelines in a specific pathogen-free facility. C57BL/6 wild type mice were purchased from Simonsen Laboratories (Gilroy, CA). Foxp3-DTR mice were purchased from The Jackson Laboratories (Bar Harbor, Maine). Foxp3cre and EGFRfl/fl mice on C57BL/6 background were kindly provided by Dr. Jeffrey Bluestone (University of California San Francisco) and Dr. David Threadgill (University of North Carolina at Chapel Hill), respectively.
Skin wounding assays and analysis
Six full-thickness excisional wounds were generated with a 4-mm sterile punch (Stiefel Laboratories, Research Triangle Park, NC) after depilation. Wounds were photographed and wound area measured with image analysis software (ImageJ 1.47v). The surface area of wound defects was expressed as a percentage of closure, relative to the initial surface of each wound.
In vivo Treg ablation
Foxp3-DTR and control mice were injected i.p. with DT (Sigma), at 30 ng/g body weight according to three regimens. For prolonged Treg depletion, mice were injected with DT on day -2 and day -1 prior to wounding as well as every other day after wounding until day 11 post-wounding. For ‘early’ and ‘late’ Treg depletion, mice received a total of 5 injections, starting either two days before (day -2 to day 5) or 5 days after (day 5 to day 10) wounding.
RESULTS & DISCUSSION
Tregs facilitate normal skin repair
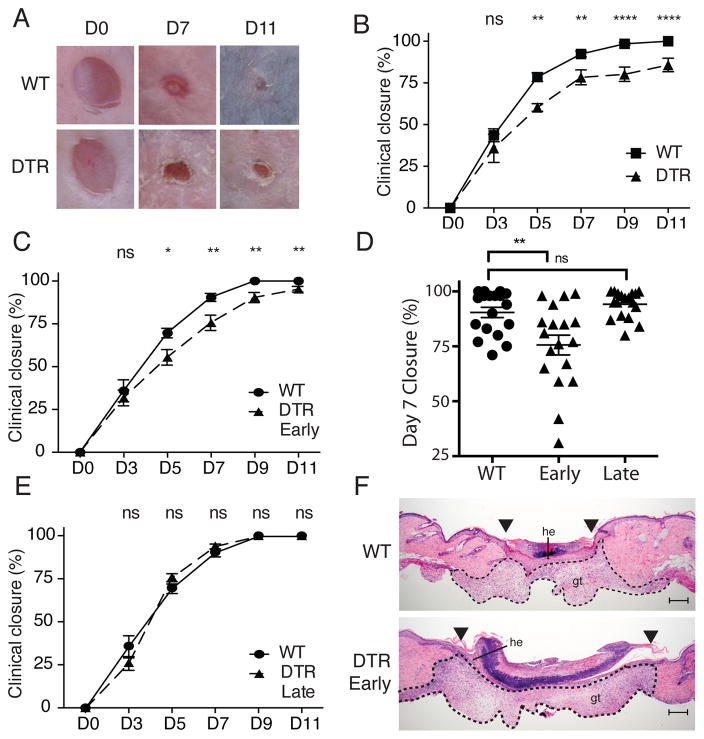
Restoration of barrier integrity following trauma is a critical and highly conserved function of skin. Response to skin injury occurs in three overlapping stages: inflammatory, new tissue formation, and remodeling (7). The inflammatory phase occurs early after wounding and is characterized by an abrupt activation of the innate and adaptive immune systems. Because Tregs constitute a large percentage of lymphocytes that reside in skin (1, 2), and because these cells play a major role in regulating tissue inflammation, we set out to determine if Tregs play a role in cutaneous wound healing. Mice transgenic for the diphtheria toxin receptor under the control of the Foxp3 promoter (Foxp3-DTR) allow for robust deletion of Tregs following administration of Diphtheria toxin (DT) (8). In initial experiments, Foxp3-DTR or wild-type (WT) mice were treated with DT for 2 days to deplete Tregs prior to full thickness wounding on dorsal skin. After wounding, mice were continuously treated with DT (every 2 days) to maintain Treg depletion, and the size of the skin defect was measured clinically and histologically over time. Wounded mice depleted of Tregs had significantly delayed kinetics of wound closure when compared to WT mice treated with DT (Fig. 1A and B). Whereas all wounds in control animals were completely closed (100% closure) between 9 and 11 days after wounding, only 75% closure was observed at this time in Treg-depleted mice, with some wounds never completely closing during the study period (Fig. 1B).
Figure 1. Tregs facilitate skin wound repair.
Foxp3-DTR or WT mice were treated with DT 2 days prior to full thickness wounding of dorsal skin and every 2 days thereafter. (A) Representative images of wounds at specific times after injury (D, day). (B) Mean percentage of wound closure with time after injury. (C) Mean percentage of wound closure with time after injury between WT and Foxp3-DTR mice treated with DT “early” after wounding. (D) Representative plot of mean percentage of wound closure 7 days after wounding. Each symbol represents an individual wound. (E) Percent of wound closure with time after injury between WT and Foxp3-DTR mice treated with DT ‘late’ after wounding. (F) Representative histology of skin wounds at day 7 post-injury between WT and Foxp3-DTR mice treated ‘early’ with DT. Arrowheads denote wound edges; he, hypertrophic epithelium; gt, granulation tissue; scale bars, 200μm. Representative data is shown from ≥ 3 replicate experiments with ≥ 3 mice per group. Error bars in all panels represent the mean ± SEM. ns p>0.05, * p≤0.05, ** p≤0.01, *** p≤0.001, **** p≤0.0001.
To determine when Tregs are required to facilitate wound repair, we depleted these cells ‘early’ after wounding (during the inflammatory phase of the response) or ‘late’ after wounding (after resolution of the inflammatory phase, when new tissue formation predominates). Depletion of Tregs during the first 5 days after wounding resulted in a significant attenuation of wound closure during the days that followed (days 5 -11) (Fig. 1C), with the most pronounced difference occurring 7 days after wounding (Fig. 1D). In contrast, depleting Tregs after the inflammatory phase (i.e., from days 5 to 12) had no effect on the kinetics of wound closure (Fig. 1E). The effects of early Treg depletion were most pronounced upon histologic examination of wounded tissue. Whereas control mice showed complete re-epithelialization of the skin defect by 7 days after wounding, mice depleted of Tregs had markedly reduced kinetics of wound re-epithelialization, with small areas of keratinocyte hyperplasia present only at the wound edges (Fig. 1F). Mice depleted of Tregs also showed a trend towards increased granulation tissue and size of the overlying eschar (Fig. 1F). These results demonstrate that Tregs play a role in facilitating skin wound healing. In addition, they suggest that these cells act early after wounding, during the inflammatory phase.
Activated Tregs accumulate in wounded skin
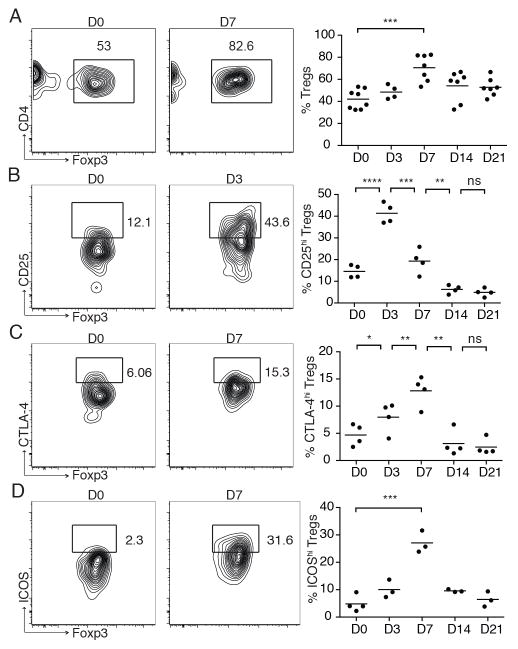
Given that Tregs facilitate skin wound repair and mediate their effects early after wounding, we set out to functionally characterize these cells in skin of wounded mice. Consistent with previous reports, we observed a marked accumulation of CD4+ T cells in wounded skin, peaking around 7 days after wounding (data not shown) (9). Interestingly, the majority of CD4+ T cells that accumulate in wounded skin are highly activated Tregs (Fig. 2). Whereas Foxp3-expressing Tregs comprise 30–50% of total CD4+ T cells in adult mouse skin in the steady state, the percentage of these cells progressively increased with time after wounding, peaking at 7 days, where they comprised approximately 70% of the total CD4+ population (Fig. 2A). In addition, the density of Tregs increased approximately 20-fold in skin by 7 days after wounding (mean number of Tregs per cm2 of skin at baseline vs. day 7 after wounding = 12 ± 2 vs. 279 ± 65, p=0.0003). Treg accumulation in wounded skin is primarily a result of migration from secondary lymphoid organs, as treatment of wounded mice with FTY720 (which blocks lymphocyte egress from lymphoid organs) resulted in a significant reduction of these cells in skin at days 3 and 7 after wounding (Supplemental Figure 1). Tregs accumulating in wounded skin had a highly activated phenotype, with increased percentages of cells expressing high levels of CD25, CTLA-4 and ICOS with time after wounding (Fig. 2B–D). Increased expression of CD25 preceded increases in CTLA-4 and ICOS expression, suggesting that IL-2 may play a role in Treg activation early after injury. Taken together, these results demonstrate that Tregs are activated and preferentially accumulate in skin during the inflammatory phase of wound healing, and that depleting these cells during this phase attenuates normal wound closure.
Figure 2. Activated Tregs accumulate in wounded skin.
Full thickness wounds were introduced to the dorsal skin of WT mice and skin-infiltrating CD4+ T cells were assayed at specific time points by flow cytometry. (A) Representative flow cytometric plots and percentage of Tregs in skin with time after wounding. Pre-gated on live CD45+CD3+CD4+ cells. (B, C, D) Percent of skin Tregs that express high levels of CD25, CTLA-4, and ICOS with time after wounding. Pre-gated on live CD45+CD3+CD4+Foxp3+ cells. Each symbol represents an individual mouse. Bars represent means. Representative data is shown from ≥ 3 replicate experiments with ≥ 3 mice per group. ns p>0.05, * p≤0.05, ** p≤0.01, *** p≤0.001, **** p≤0.0001.
Tregs suppress IFNγ production and pro-inflammatory macrophage accumulation in wounded skin
Quantitative and/or qualitative defects in macrophages significantly inhibit skin wound healing. Macrophage depletion early after wounding results in impaired granulation tissue formation and wound re-epithelialization (10). In addition, the retention and persistence of pro-inflammatory macrophages in skin significantly attenuates wound closure in mouse models and is a hallmark of non-healing skin ulcers in humans (11). Interestingly, Tregs have been shown to influence macrophage function in muscle (6). Thus, we hypothesized that a major role of Tregs in wounded skin is to regulate macrophage polarization during the inflammatory phase of wound repair. To test this, we depleted Tregs early after wounding and assessed macrophage polarization. Pro-inflammatory macrophages (as defined by CD45+CD11bhighF4/80+Ly-6ChighLy-6GlowCD206low) accumulated in skin early after wounding, peaking at 24 hours and gradually declining to background levels by 14 days (Fig. 3A). Depletion of Tregs augmented accumulation of pro-inflammatory macrophages in wounded skin (Fig. 3B). There were significantly higher percentages (Fig 3B) and absolute numbers (data not shown) of pro-inflammatory macrophages in the skin of Treg-depleted mice early after wounding (day 3), which translated to increased persistence of these cells during the entire course of wound closure.
Figure 3. Tregs limit the accumulation of IFNγ-producing T cells and pro-inflammatory macrophages in wounded skin.
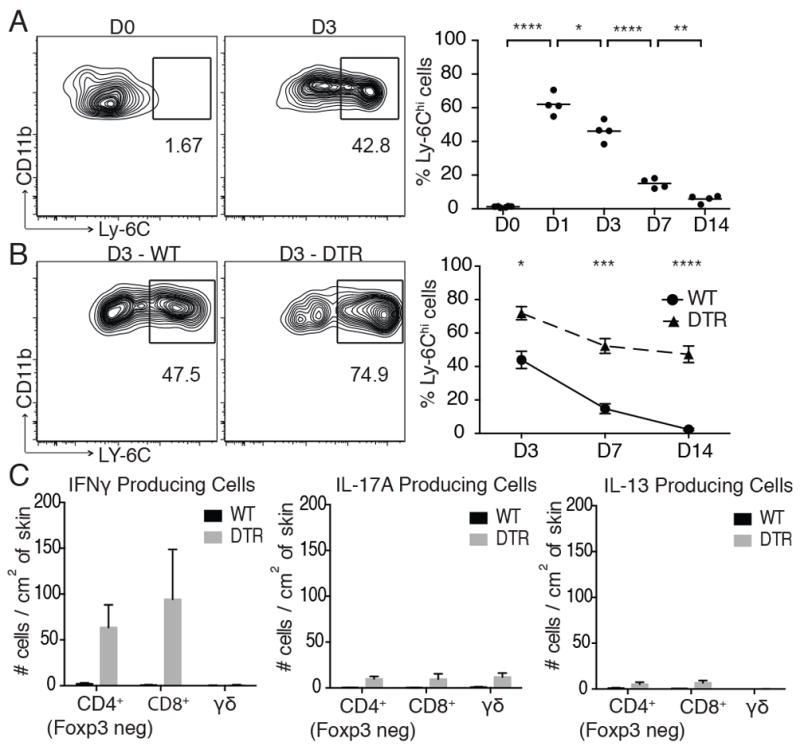
(A) Full thickness wounds were introduced to the dorsal skin of WT mice and the percentage of skin-infiltrating pro-inflammatory macrophages were assayed at specific time points by flow cytometry. (B) Foxp3-DTR or WT mice were treated with DT ‘early’ after wounding and percentage of skin-infiltrating pro-inflammatory macrophages were assayed at specific time points by flow cytometry. (C) Absolute number of cytokine producing T cell subsets in skin 7 days after wounding in DT-treated Foxp3-DTR or WT mice as measured by intracellular cytokine staining using flow cytometry. Representative data is shown from ≥ 3 replicate experiments with ≥ 3 mice per group. Error bars in all panels represent the mean ± SEM. ns p>0.05, * p≤0.05, ** p≤0.01, *** p≤0.001, **** p≤0.0001.
Interferon gamma is a major driver of pro-inflammatory macrophage differentiation and accumulation (13). In addition, Tregs suppress IFNγ production from T cells in skin (14). Thus, we speculated that increased pro-inflammatory macrophages observed in Treg-depleted mice would correlate with high levels of IFNγ-producing T cells infiltrating wounded skin. To test this, we treated Foxp3-DTR or WT mice with DT and quantified skin T cell subsets and relative cytokine production from these cells after wounding. Depletion of Tregs resulted in a marked increase of both CD4+ and CD8+ T cells infiltrating wounded skin, with little change in dermal γδTCR+ cells, a T cell population resident in normal mouse skin (15) (data not shown). Low numbers of cytokine producing T cells were observed in wounded skin of WT mice; however, there was a pronounced increase in cytokine producing cells upon depletion of Tregs, with the overwhelming majority of these cells producing IFNγ, relative to IL-17A and IL-13 (Fig. 3C). Consistent with a role of IFNγ in driving pro-inflammatory macrophage accumulation, in vivo neutralization of IFNγ resulted in a significant reduction in the accumulation of these cells in skin of Treg-depleted mice after wounding (Supplemental Fig. 2)
These results suggest that a major function of Tregs in the context of skin injury is to limit the accumulation of IFNγ-producing T cells and pro-inflammatory macrophages in wounded skin. This is consistent with studies showing accelerated wound healing in mice genetically deficient in IFNγ (16). We speculate that the persistence of pro-inflammatory macrophages in Treg-depleted mice is a major contributor to the delay in wound healing observed in these animals. Our findings add to a growing body of literature defining the role of Tregs in regulating macrophage function in tissues.
The EGFR pathway is required for Treg function in wounded skin
Given that the EGFR pathway plays a role in both wound healing and T cell function (3–5), we set out to determine whether this pathway participates in the ability of Tregs to attenuate wound-associated inflammation and facilitate skin repair. To this end, we first characterized EGFR expression on SDLN and skin Tregs purified from Foxp3-reporter (Foxp3-DTR) mice. Because robust antibodies to murine EGFR are not available for flow cytometry, we performed qRT-PCR for EGFR expression on cells isolated from SDLN and skin before and after wounding. Expression of EGFR was not detected in Foxp3+ or Foxp3− CD4+ T cells isolated from skin or SDLNs prior to wounding (data not shown). However, a marked induction of EGFR expression was detected in skin Tregs 3 days after wounding (Fig. 4A). Interestingly, EGFR expression was not observed in SDLN Tregs at any time post-wounding, suggesting that induction of EGFR occurs preferentially on Tregs in inflamed skin in our model.
Figure 4. The EGFR pathway is required for Treg function in wounded skin.
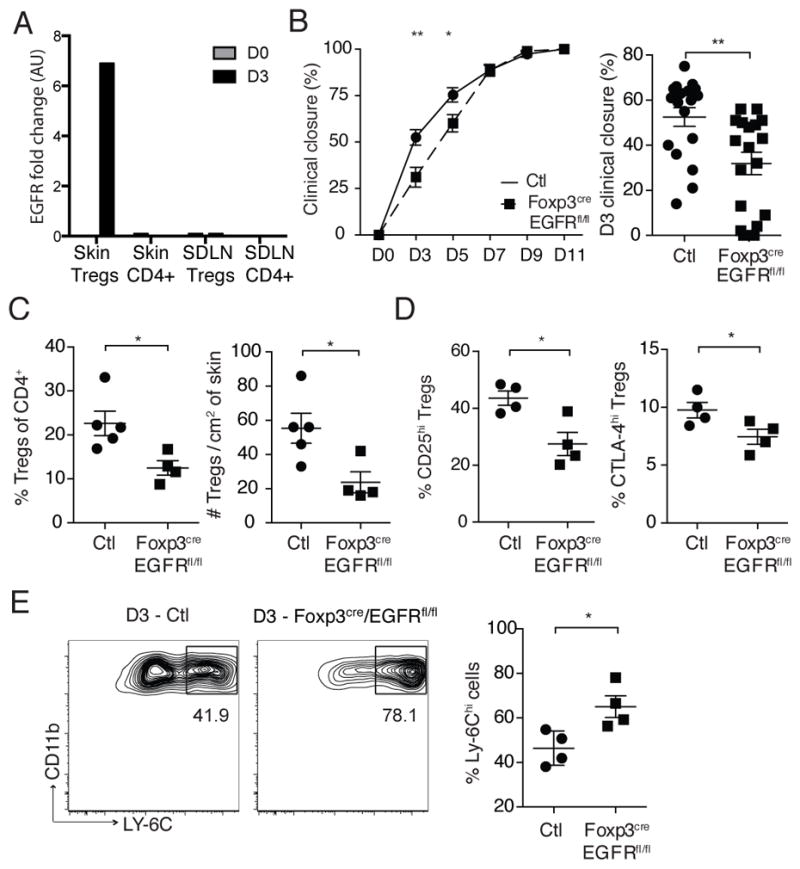
(A) Foxp3-expressing and non-expressing CD4+ T cells were purified from skin and skin draining lymph nodes (SDLN) of Foxp3 reporter (Foxp3-DTR) mice. qRT-PCR for EGFR was performed on purified cells 3 days after full thickness wounding. Results are fold change (FC) relative to β2microglobulin (B2M). (B) Mean percentage of wound closure and a representative plot of percentage of wound closure for individual wounds 3 days after injury between Foxp3creEGFRfl/fl and control (Ctl) mice. Control mice represent either WT or Foxp3creEGFRwt/wt mice. (C) Percent and absolute number of Tregs in skin 3 days after wounding. Pre-gated on live CD45+CD3+CD4+ cells. (D) Percent of Tregs that express high levels of CD25 and total CTLA-4 3 days after wounding. (E) Percentage of skin-infiltrating pro-inflammatory macrophages 3 days after wounding as assayed by flow cytometry. Pre-gated on CD45+CD11bhighF4/80+Ly-6Glowcells. Representative data is shown from ≥ 3 replicate experiments with ≥ 4 mice per group. Error bars in all panels represent the mean ± SEM. AU, arbitrary units. ns p>0.05, * p≤0.05, ** p≤0.01
To determine if EGFR expression on Tregs plays a functional role in their ability to facilitate wound repair, we bred Foxp3cre mice (17) with EGFRfl/fl mice (18) to generate animals with a conditional deletion of EGFR only in Tregs (Foxp3creEGFRfl/fl). Consistent with the absence EGFR expression on Tregs in the steady state, adult Foxp3creEGFRfl/fl mice had similar percentages of Tregs in the skin and SDLNs when compared to age- and gender-matched WT mice (data not shown). In addition, the basal activation of Tregs was similar between Foxp3creEGFRfl/fl mice and WT mice in the steady-state, and Foxp3creEGFRfl/fl mice do not develop de novo skin inflammation (data not shown). Interestingly, Foxp3creEGFRfl/fl mice had significantly delayed kinetics of wound closure when compared to either Foxp3creEGFRwt/wt or WT controls (Fig. 4B). The effects of the EGFR pathway in Tregs was observed early after wounding, with attenuated wound closure seen only on days 3 and 5 post-wounding (Fig. 4B). Mechanistically, deletion of the EGFR pathway in Tregs resulted in reduced percentages and absolute numbers of these cells in skin early after wounding (Fig. 4C). In addition, Tregs in skin of wounded Foxp3creEGFRfl/fl mice were less activated, with reduced percentages of cells expressing high levels of CD25 and CTLA-4 (Fig. 4C). Consistent with a role for Tregs in attenuating inflammatory macrophages after wounding, Foxp3creEGFRfl/fl mice had increased percentages of pro-inflammatory macrophages early after wounding (Fig. 4E).
Taken together, our data support a role for Tregs in attenuating wound-associated inflammation and facilitating skin wound healing. These cells primarily mediate their effects during the inflammatory phase of wounding and EGFR expression on Tregs plays a role, at least in part, for their regulatory effects in this context. Preliminary data from our laboratory suggests that EGFR expression on Tregs may act to enhance Treg survival during inflammation (data not shown). Our results add to an emerging body of work showing that the EGFR pathway is important for Treg function in tissues. The EGFR ligand, AREG, is produced by Tregs that infiltrate injured muscle and lung, where it plays a role in enhancing tissue repair (4, 5). In contrast, activated Tregs in mice and humans express EGFR, and signaling through this receptor enhances their suppressive capacity in vitro (6). Our data is consistent with the notion that Tregs increase EGFR expression in vivo upon induction of tissue inflammation and that signaling through this receptor enhances their function. However, the fact that EGFR deletion in Tregs has a lesser effect when compared to specific ablation of the entire cell subset (i.e., Foxp3-DTR mice), suggests that Tregs utilize more than just the EGFR pathway to mediate their functions in facilitating wound repair.
In skin wound healing, current dogma suggests that the EGFR pathway mediates it effects on non-lymphoid parenchymal cells such as fibroblasts, keratinocytes and endothelial cells. However, our data suggests that this pathway also augments Treg function. It is interesting to speculate that the adaptive immune system has co-opted this highly conserved and vital pathway in skin to regulate tissue inflammation, in an attempt to facilitate proper repair. Therapeutic approaches to treat chronic skin ulcers have focused on augmentation of EGFR signaling (3). Our data suggest that these strategies may work in part by augmenting cutaneous Treg function. Conversely, ineffective Tregs may predispose to chronic non-healing ulcers. Thus, local therapeutic manipulation of Tregs may be a novel strategy to treat wound-associated inflammation with the potential to expedite healing.
Supplementary Material
Acknowledgments
We thank C. Benetiz for assistance with animal husbandry.
Grant Support: This work was primarily funded by M.D.R. grants: Scleroderma Research Foundation Grant, NIH K08-AR062064, Burroughs Wellcome Fund CAMS-1010934, NIH R21-AR066821, NIH DP2-AR068130 and a National Psoriasis Foundation Translational Grant. Flow Cytometry data was generated in the UCSF Parnassus Flow Cytometry Core which is supported by the Diabetes Research Center (DRC) grant, NIH P30 DK063720. Histology was performed with assistance from the UCSF Mouse Pathology Core which is supported by NIH 5P30CA082103-15. A.N. was supported by: René Touraine Foundation, Philippe Foundation and SIV.
References
- 1.Gratz IK, Truong HA, Yang SHY, Maurano MM, Lee K, Abbas AK, Rosenblum MD. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol Baltim Md 1950. 2013;190:4483–4487. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, Yang SH-Y, Anthony BA, Sverdrup FM, Krow-Lucal E, MacKenzie TC, Johnson DS, Meyer EH, Löhr A, Hsu A, Koo J, Liao W, Gupta R, Debbaneh MG, Butler D, Huynh M, Levin EC, Leon A, Hoffman WY, McGrath MH, Alvarado MD, Ludwig CH, Truong H-A, Maurano MM, Gratz IK, Abbas AK, Rosenblum MD. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124:1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodnar RJ. Epidermal Growth Factor and Epidermal Growth Factor Receptor: The Yin and Yang in the Treatment of Cutaneous Wounds and Cancer. Adv Wound Care. 2013;2:24–29. doi: 10.1089/wound.2011.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaiss DMW, van Loosdregt J, Gorlani A, Bekker CPJ, Gröne A, Sibilia M, van Bergen en Henegouwen PMP, Roovers RC, Coffer PJ, Sijts AJAM. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Mehta ND, Zhao Y, DiPietro LA. Absence of CD4 or CD8 lymphocytes changes infiltration of inflammatory cells and profiles of cytokine expression in skin wounds, but does not impair healing. Exp Dermatol. 2014;23:189–194. doi: 10.1111/exd.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol Baltim Md 1950. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 11.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkötter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, Margeta M, Spencer MJ, Bluestone JA. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med. 2014;6:258ra142. doi: 10.1126/scitranslmed.3009925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenblum MD, I, Gratz K, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol Baltim Md 1950. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol Baltim Md 1950. 2004;172:1848–1855. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee TC, Threadgill DW. Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genes N Y N 2000. 2009;47:85–92. doi: 10.1002/dvg.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.