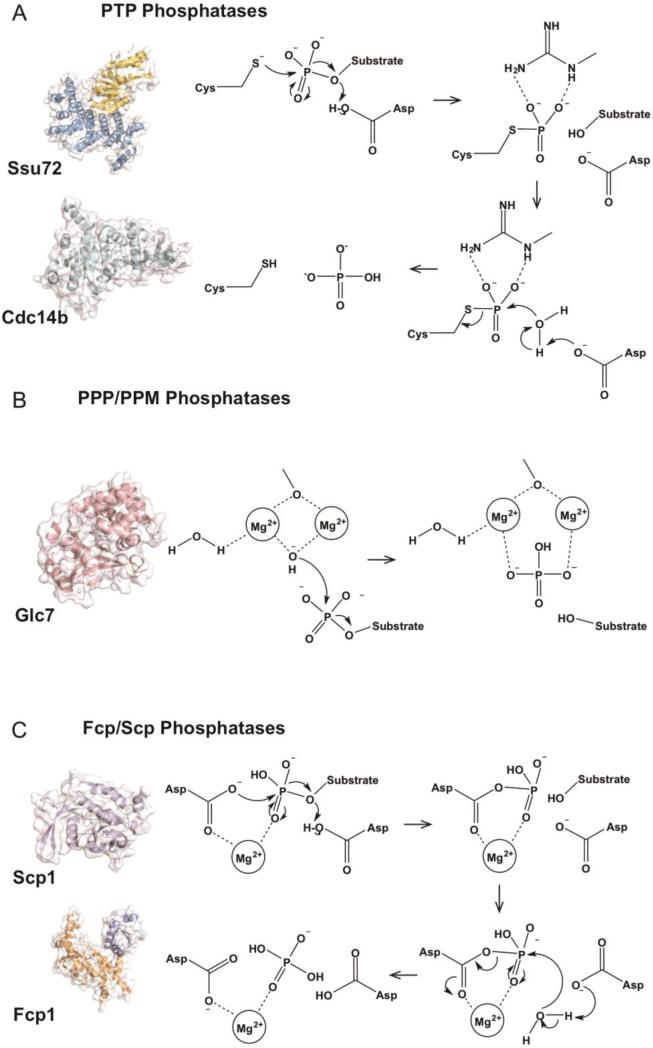
Figure 2.
CTD phosphatase mechanisms. A) PTP family phosphatase mechanism. PTP phosphatases utilize a cysteine nucleophile to generate a cysteinyl-phosphate intermediate. This intermediate is then resolved utilizing active site water. This mechanism is utilized by CTD phosphatases Ssu72 (Ssu72 in yellow, Symplekin in blue, D. melanogaster, PDB ID: 4IMJ) and Cdc14b (cyan, human, PDB ID: 1OHC). B) PPP/PPM family phosphatase mechanism. PPP/PPM phosphatases utilize a di-metal mechanism to coordinate and activate a water molecule that acts as nucleophile and liberates phosphate from substrate. This mechanism is utilized by Glc7 (salmon, human PP1 (86% identity), PDB ID: 4MOV). C) Fcp/Scp family phosphatase mechanism. Fcp/Scp phosphatases (Fcp1 orange and blue, S. pombe, PDB ID: 3EF0; Scp1 in purple, human, PDB ID: 2GHT) utilize a conserved DXDX(T/V) motif and magnesium ion to coordinate phosphorylated serine. Aspartic acid acts as a nucleophile and forms an aspartyl-phosphate intermediate that is subsequently resolved by active site water.