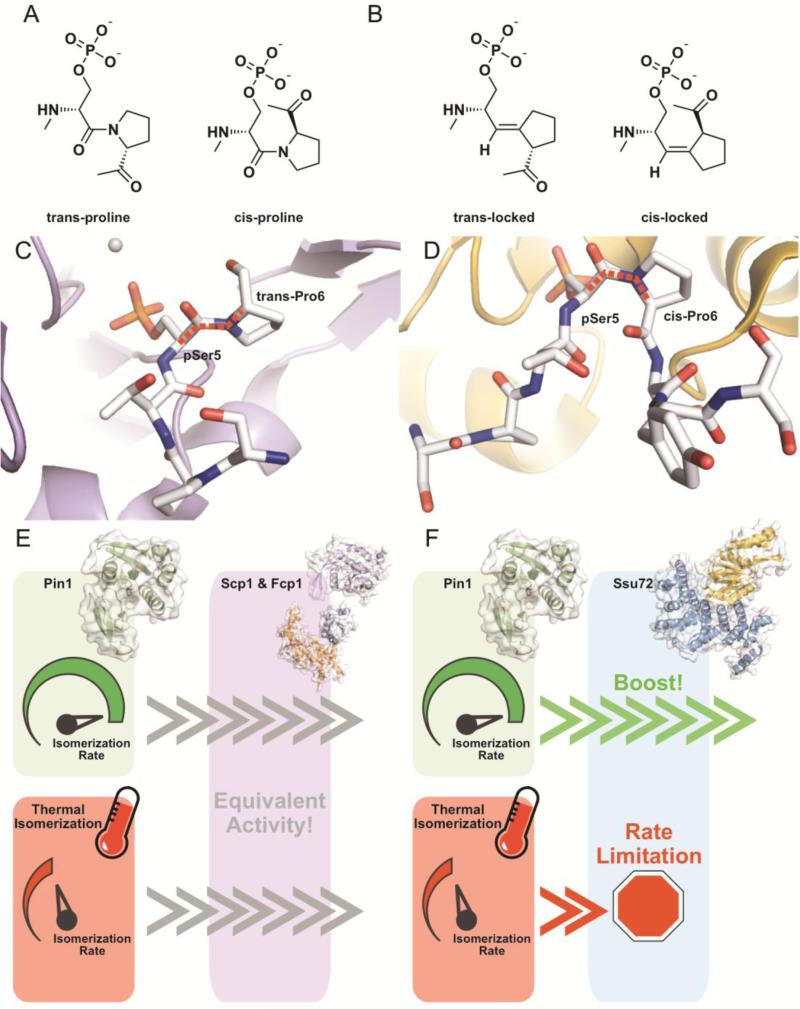
Figure 6.
Specific enhancement of Ssu72 activity by proline isomerization. A) The heptad repeat of the CTD is highly enriched with phospho-serine/proline motifs. The proline of these motifs is unique among natural amino acids in its ability to stably assume both a trans (left) and cis (right) conformation about its prolyl peptide bond. B) A chemical biology approach was used to generate phospho-Ser5 CTD peptides containing trans- (left) and cis-locked (right) proline. The prolyl-peptide bond is replaced with a carbon-carbon double bond that does not undergo thermal isomerization. These chemical tools allow for the investigation of proline isomer preference of proteins known to recognize phospho-Ser5 in the CTD, like CTD phosphatases Ssu72 and Scp1. C) The human Scp1 (Figure C, purple and white, PDB ID: 2GHT) and D) D. melanogaster Ssu72 (Figure D, yellow and white, PDB ID: 4IMJ) structures provide clues to proline isomer preference. Pro6 was found in the trans conformation in the Scp1/pSer5 peptide complex structures suggesting either trans proline preference or no inherent preference. Pro6 was resolved in the cis conformation in Ssu72/pSer5 peptide complex structures suggesting a cis-proline preference. Isomerized bonds are highlighted with a dashed red line. E) Trans-specific or preferred phosphatases like Scp1 and Fcp1 (Scp1 in purple, human, PDB ID: 2GHT; Fcp1 orange and blue, S. pombe, PDB ID: 3EF0) bypass regulation by proline isomerization state due to their preference for the major proline species and have equivalent apparent activity with or without prolyl isomerases. F) In vitro and cellular experiments demonstrate Ssu72 (Ssu72 in yellow, Symplekin in blue, D. melanogaster, PDB ID: 4IMJ) activity is enhanced by the presence of phospho-specific prolyl isomerase Pin1 (green, human, PDB ID: 2Q5A) due to its structural requirement for cis-proline. The activity of prolyl isomerases boosts the apparent dephosphorylation of CTD by Ssu72, while thermal isomerization of prolines in CTD becomes rate limiting and leads to an accumulation of phospho-Ser5 CTD in the cell.