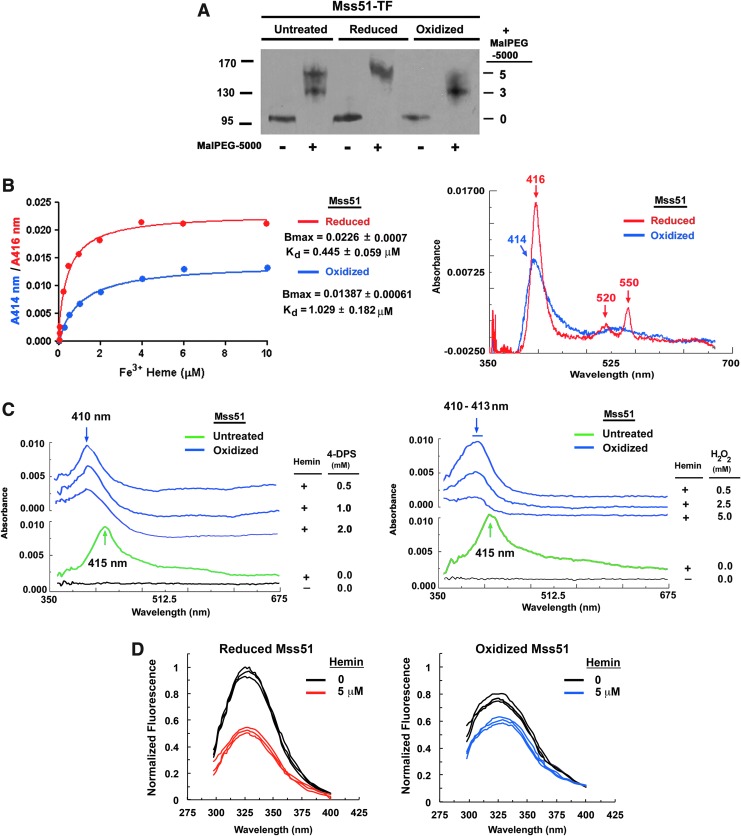
FIG. 2.
The redox state of recombinant Mss51-TF determines hemin coordination. (A) Mss51-TF redox state in vitro by thiol trapping. Purified recombinant Mss51-TF aliquots were left untreated, reduced (10 mM dithiothreitol), or oxidized (1 mM 4-DPS) and subsequently exposed to Mal-PEG5000. Proteins were resolved in SDS-PAGE under nonreducing conditions and Mss51 was detected by immunoblotting. (B) Difference spectroscopy titration of hemin binding to reduced or oxidized Mss51-TF performed with increasing concentrations of Fe3+ hemin. The titration curves (left panel) were generated from fits to equation, 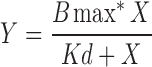 , describing a single binding site using GraphPad Prism software. The right panel displays the overlay of titration curves showing peaks, maxima, after binding 2 μM hemin. (C) Difference absorption spectra of hemin-bound native Mss51-TF treated with increasing concentrations of 4-DPS (left panel) and H2O2 (right panel), as shown. The results were obtained and analyzed as explained in (B). (D) Fluorescence emission spectra of reduced and oxidized Mss51-TF upon binding to hemin. Spectra were recorded at 25°C in 50 mM Tris-HCl pH 8.0 (excitation at 295 nm). The fluorescence maximum of reduced Mss51-TF was arbitrarily set to 1 fluorescence unit. For each condition, plots from three independent samples are presented. 4-DPS, aldrithiol-4; redox, reduction–oxidation; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
, describing a single binding site using GraphPad Prism software. The right panel displays the overlay of titration curves showing peaks, maxima, after binding 2 μM hemin. (C) Difference absorption spectra of hemin-bound native Mss51-TF treated with increasing concentrations of 4-DPS (left panel) and H2O2 (right panel), as shown. The results were obtained and analyzed as explained in (B). (D) Fluorescence emission spectra of reduced and oxidized Mss51-TF upon binding to hemin. Spectra were recorded at 25°C in 50 mM Tris-HCl pH 8.0 (excitation at 295 nm). The fluorescence maximum of reduced Mss51-TF was arbitrarily set to 1 fluorescence unit. For each condition, plots from three independent samples are presented. 4-DPS, aldrithiol-4; redox, reduction–oxidation; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.