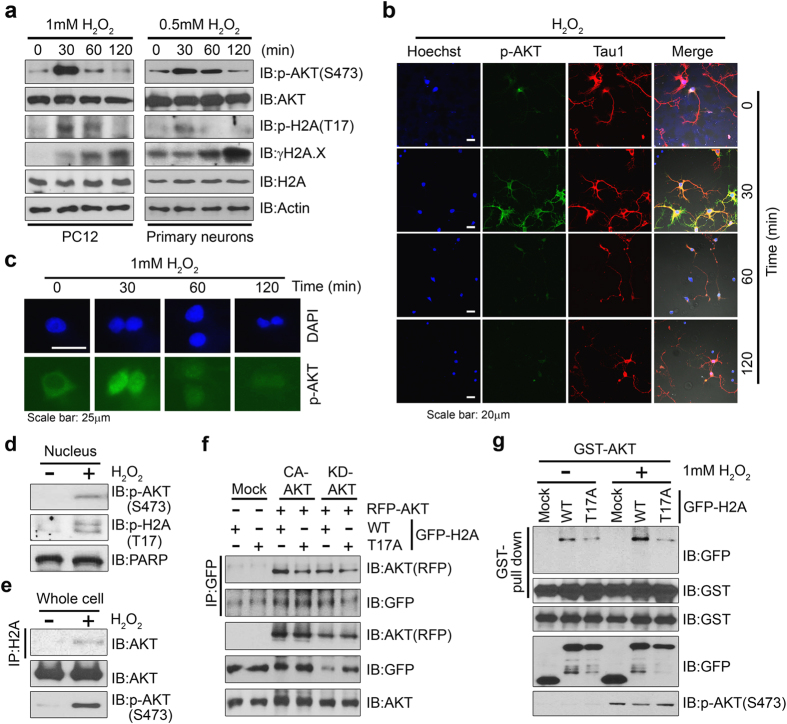
Figure 3. Phosphorylation of H2A on T17 by Akt occurs during H2O2-induced cell death.
(a) PC12 cells (left) and primary cultured hippocampal neurons (right) were treated with 1 mM or 0.5 mM H2O2 respectively for 30, 60, and 120 min and immunoblotting was performed with the indicated antibodies. (b) Fixed hippocampal neuron cells were stained with Hoechst 33342 (blue), anti-pAkt antibody (green), and anti-Tau1 antibody (red). (c) PC12 cells were maintained without serum for 4 h, followed by H2O2 treatment. Fixed cells were stained with DAPI (blue) and anti-pAkt antibody (green). After H2O2 stimulation for 30 min, active Akt translocated from the cytoplasm into the nucleus. (d) Total nuclear proteins were isolated from PC12 cells treated with or without H2O2 and fractionated into cytoplasmic and nuclear proteins. Nuclear fractions were analyzed by immunoblotting with anti-pH2A antibody. PARP was used as a marker for the nuclear fraction. (e) PC12 cells were stimulated with 1 mM H2O2 for 30 min and cell lysates were used for immunoprecipitation with anti-H2A antibody. The association between endogenous Akt and H2A was enhanced by H2O2 treatment. (f) RFP-Akt constructs (active Akt T308DS473D or kinase-dead K179A) were co-transfected with GFP-tagged H2A-WT and mutant (H2A-T17A) into HEK 293T cells. Cell lysates were analyzed by immunoblotting with the indicated antibodies. (g) GST-Akt and GFP-H2A constructs (WT and mutant H2A-T17A) were co-transfected into PC12 cells. After 24 h, cells were stimulated with 1 mM H2O2 for 30 min and cell lysates were used for co-precipitation with glutathione beads. The association between exogenous Akt and H2A-WT was enhanced by H2O2 treatment.