Abstract
Hymenoscyphus fraxineus, an introduced ascomycete fungus and primary causal agent of European ash dieback, was investigated on Fraxinus mandshurica trees in its native range in Primorye region of Far East Russia. This evidence is the first report of H. fraxineus on healthy, asymptomatic F. mandshurica trees. High-throughput sequencing revealed 49 distinct fungal taxa associated with leaves of F. mandshurica, 12 of which were identified to species level. Phyllosphere fungal assemblages were similar among sites despite being largely geographically distant. Many organisms comprising the foliar fungal community on F. mandshurica in Far East Russia have similarity to those reported inhabiting F. excelsior in Europe based on previous studies. However, Mycosphaerella sp., the most dominant species in this study and detected in nearly all samples, was associated only with F. mandshurica. Genetic diversity of H. fraxineus was significantly higher in the Far East Russian population than in Europe. In contrast to its aggressive behaviour on Fraxinus excelsior in Europe, H. fraxineus appears to be a benign associate of indigenous F. mandshurica that initially induces quiescent and asymptomatic infections in healthy trees prior to active host colonization normally associated with modification of host tissue during senescence.
A new alien invasive disease affecting common ash (Fraxinus excelsior) trees has resulted in severe dieback and mortality throughout much of the natural distribution range of ash in northern and central Europe. The causal agent of disease was first described as Chalara fraxinea, characterized by brown phialides producing conidia in slimy spore droplets, and occasionally in chains1. Its teleomorph was mistakenly recognized as Hymenoscyphus albidus, a discomycete native to Europe that produces numerous white-stalked apothecia on fallen rachises, and which had never been reported to be pathogenic2. Differences in the genomic DNA markers internal transcribed spacer (ITS), elongation factor-1-α (EF1-α), and calmodulin (CAL) gene and inter simple sequence repeat-polymerase chain reaction (ISSR-PCR) fingerprinting of specimens collected from diseased and healthy ash stands clearly delimited two species that appeared morphologically indistinct3. The separate cryptic telemorph species associated with the anamorph C. fraxinea was subsequently named Hymenoscyphus pseudoalbidus3. Subsequent work has demonstrated subtle microscopic differences between the two Hymenoscyphus species on the basis of presence or absence of ascus croziers4. Recently, a suggested renaming of the fungus to H. fraxineus Baral, Queloz, Hosoya was proposed5.
Apothecia of H. fraxineus are produced during the summer mainly on blackened pseudosclerotial rachises from the previous year in the leaf litter6,7,8. Occasionally apothecia have been noted also on small ligneous stems2,7. Following spore dispersal during several weeks in the summer, germ tube formation and development of appressorium facilitate penetration of the ash leaf cuticle8 and necrosis expands proximally preferentially along leaf veins to rachises and subsequently twigs and branches leading to a wide range of symptoms including wilting of shoots, bark cankers, wood discoloration and dieback of twigs, branches and the crown9,10.
Population studies of H. fraxineus in Europe show high genotypic diversity11,12 suggesting an outbreeding mating system and long-range dispersal via ascospores, and reported low allelic richness and low differentiation among European populations of H. fraxineus13,14,15. Collectively the different studies conducted throughout Europe suggest no population structure, and that the pathogen must have gone through a strong genetic bottleneck in the European populations12,13,14,15, with the exception of some differentiation between the Polish highland and lowland populations11. H. fraxineus in Europe is conspecific with Japanese specimens16, previously known under the name Lambertella albida (≡ H. albidus) collected from decaying rachises of F. mandshurica var. japonica. The Asian H. fraxineus population showed higher genetic diversity and was genetically differentiated from the European population17 suggesting the origin of the pathogen introduced to Europe is likely East Asia.
In Russia, there is large geographic separation between natural distribution ranges of F. excelsior in the west and Manchurian ash (F. mandshurica) in the east. In Russia’s Kaliningrad district, which is approximately in the far eastern range of F. excelsior’s natural distribution, H. fraxineus is well established having spread from diseased areas in nearby Baltic countries, and is causing decline and mortality of trees18. Fraxinus sogdiana, a species of middle Asia, was shown to be susceptible to H. fraxineus in Krussmann’s ash belt in Central Asia19. In Far East Russia, F. mandshurica grows in natural mixed forests of Primorye region which represents the north-eastern and part of the central area of the disjunctive natural range of the species in the East20. The ecology of H. fraxineus on F. mandshurica and other Fraxinus species within its native environment in East Asia however still remains unclear. Contrasting reports of disease expression exist between amenity plantings of F. mandshurica within the current zone of infestation in Europe and that within its native range in the East. First, there have been no reports of leaf symptoms on F. mandshurica in its native range of Asia that would suggest H. fraxineus is a pathogen on F. mandshurica. Yet following artificial stem-wound inoculations on seedlings, H. fraxineus was shown to be pathogenic on F. mandshurica21. In Estonia, typical symptoms of ash dieback were reported on 40-year-old F. mandschurica planted in a park and H. fraxineus was consistently isolated from shoots of trees displaying these symptoms22, though it was noted that among the non-native Fraxinus species, F. mandshurica and F. americana were the least affected with only minor shoot and twig dieback and bark necrosis. The above reports21,22 contrast with surveys of a number of Asian Fraxinus species, including F. mandshurica, planted alongside severely damaged F. excelsior in southern Swedish arboreta (Alnarp, Göteborg) which showed no wilting, bark necrosis or dieback of twigs and branches (Fig. 1) (M. Cleary, unpublished data). Similar observations of disease-free Asian Fraxinus have been reported from arboreta in Denmark23. H. fraxineus is found on shed pseudosclerotial rachises of F. mandshurica, and its suggested role is as a decomposer of ash leaves in the leaf litter16. Since pathogenicity to indigenous Fraxinus species in the East has so far not been reported under natural conditions, one may speculate that H. fraxineus in its native environment has a primary role as a saprotroph of senesced leaf tissue, but possesses a prolonged period of endophytic growth prior to saprobic colonization of host tissue normally associated with senescence.
Figure 1.
(a) Fraxinus mandshurica planted in Southern Sweden showing no evidence of crown dieback; (b) chronic and progressive dieback symptoms including epicormics branching along the stem of Fraxinus excelsior trees infected by Hymenoscyphus fraxineus in Southern Sweden. Photos taken by Michelle Cleary.
Phyllosphere fungal species include both epiphytes (organisms living on the host surface) and endophytes. The vast majority of plants in natural ecosystems are colonized by endophytes which generally reside asymptomatically in apoplastic spaces and/or within the living cells of plants for all or at least a part of their life cycle by means of quiescent infections24. Phyllosphere fungi have diverse roles influencing plant fitness either negatively as pathogens25, or conferring benefits on their hosts by increasing plant’s tolerance to stress26, reducing herbivory through the production of toxic alkaloids27, and via antagonistic effects that reduce infection of plant tissues by pathogens28. Such roles are of course dependent on any large number of variables including the affected host plant species and environmental parameters that condition the host for infection. Earlier studies have revealed a large diversity of phyllosphere-inhabiting microorganisms of both healthy and diseased F. excelsior9,10,29,30,31,32 and profile a range of fungal assemblages for the species. However, little is known of the fungal community associated with F. mandshurica, whether large qualitative differences exist between F. excelsior in Europe and F. mandshurica in its native environment in the East, and whether this could, in part, explain differences in the behaviour of H. fraxineus on either Fraxinus species.
The aims of the current study were i) to investigate whether H. fraxineus is present on healthy (i.e. asymptomatic) leaves of F. mandshurica trees located in Far East Russia, ii) to describe the diversity and spatial variability of the associated fungal community of F. mandshurica trees, and iii) determine the specific structure of genetic diversity of H. fraxineus between populations in Far East Russia and Europe.
Results
Fungal community of F. mandshurica and detection of H. fraxineus in asymptomatic leaves
Investigation of fungal communities in asymptomatic F. mandshurica leaflets and rachises revealed the presence of 49 distinct fungal taxa, 12 of which were identified to species level based on sequence similarities with GenBank entries. 96% of the sequence reads exist in 20 of the most frequently occurring or dominant OTUs (distinct taxa) that belonged to six orders of Ascomycetes: Capinodiales, Pleosporales, Diaporthales, Helotiales, Hypocreales, Dothideales and an unknown Ascomycete; and to one order of Basidiomycetes, Tremellales (Table 1). Of these 20 most frequently occurring OTUs (see also Supplementary Information), seven were identified to the species level and 11 were identified to the genus level. Members of Capnodiales and Pleosporales dominated the community in both species richness (five species from each order) representing 79% and 12% of OTU relative abundance, respectively. Mycosphaerella sp. was the most common species in F. mandshurica, and was detected in 99% (n = 75) of samples examined, while Cladosporium sp., Phomopsis sp., and Phoma sp. were found in 84%, 80%, and 81% of samples, respectively. Hymenoscyphus fraxineus (Genbank accession no. KU234397) was detected by this method from 33% of the total number of samples examined in all three sites; individual site incidence ranged between 14 and 55% (Table 1).
Table 1. Relative abundance, putative identification of fungal taxa, and frequency of detection in leaves of Fraxinus mandshurica in Far East Russia, and comparative presence of similar-type taxa to Fraxinus excelsior in Europe.
| OTU no. | Cluster size | OTU relative abundance | BLAST check | Closest Genbank Accession No. | Max Score | Coverage | % max identity | Frequency of detection on F. mandshurica in Far East Russia (%)a | Presence on F. excelsior in Europeb |
|---|---|---|---|---|---|---|---|---|---|
| Putative taxon | |||||||||
| 0 | 25121 | 63.16 | Mycosphaerella sp. | EU167602 | 403 | 100 | 97 | 99 | |
| 1 | 4620 | 11.62 | Cladosporium sp. | KJ921876 | 444 | 100 | 100 | 84 | + |
| 5 | 1341 | 3.37 | Phomopsis sp. | HE774484 | 466 | 100 | 100 | 80 | + |
| 3 | 1201 | 3.02 | Phoma sp. | KJ921933 | 455 | 100 | 100 | 81 | + |
| 2 | 1062 | 2.67 | Alternaria alternata | KF819607 | 462 | 100 | 100 | 64 | + |
| 4 | 817 | 2.05 | Dioszegia sp. | AJ581077 | 361 | 100 | 97 | 51 | |
| 8 | 759 | 1.91 | Cryptococcus foliicola | AY557600 | 429 | 100 | 100 | 75 | + |
| 7 | 650 | 1.63 | Unknown Helotiales sp. | KF636763 | 435 | 100 | 99 | 47 | |
| 6 | 554 | 1.39 | Paraphoma sp. | JX077009 | 424 | 100 | 99 | 68 | |
| 9 | 342 | 0.86 | Diaporthe nobilis | KC343146 | 459 | 100 | 100 | 40 | + |
| 13 | 254 | 0.64 | Ramularia sp.1 | GU214690 | 411 | 100 | 98 | 53 | + |
| 15 | 244 | 0.61 | Paraconiothyrium sp. | FN868460 | 396 | 100 | 96 | 24 | |
| 11 | 187 | 0.47 | Hymenoscyphus fraxineus | KJ780099 | 440 | 100 | 100 | 33 | + |
| 18 | 181 | 0.46 | Periconia byssoides | KC954160 | 455 | 100 | 100 | 33 | + |
| 20 | 173 | 0.43 | Ramularia sp.2 | GU214692 | 399 | 100 | 97 | 35 | + |
| 14 | 153 | 0.38 | Coniozyma leucospermi | EU552113 | 440 | 100 | 98 | 45 | |
| 12 | 149 | 0.37 | Hannaella coprosmaensis | AF444485 | 398 | 100 | 100 | 36 | |
| 10 | 146 | 0.37 | Unknown Ascomycota sp. | JN120378 | 418 | 100 | 98 | 29 | |
| 21 | 90 | 0.23 | Ramularia sp.3 | JN662315 | 361 | 84 | 99 | 21 | + |
| 17 | 87 | 0.22 | Fusarium sp. | KJ589605 | 472 | 100 | 100 | 27 | + |
The nearly overlapping rarefactions curves and overlapping convex hulls in the NMDS indicated that the fungal community was similar at each site (Fig. 2). The Shannon-Wiener Diversity indices of the foliar fungal communities were 2.92, 2.72 and 2.83 for sites 1, 2 and 3, respectively.
Figure 2.
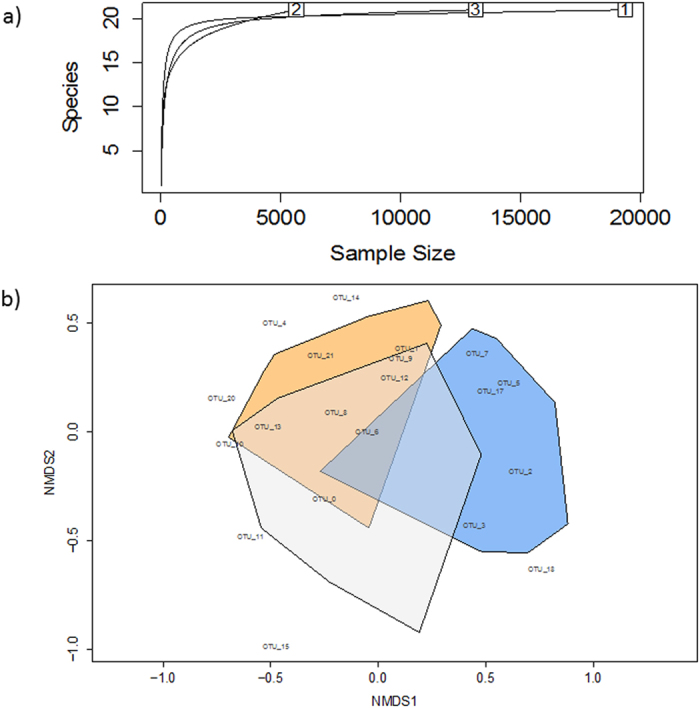
(a) Species rarefaction curve and (b) nonmetric multidimensional scaling (NMDS) ordination of the top 20 fungal OTUs on F. mandshurica at three locations. In (a), each boxed number refers to the location of each tree. In (b), each convex hull connects the vertices of the points made by each sample from each of the three locations. Orange, blue and grey colors represent individual sites/trees 1, 2, and 3, respectively. The first two dimensions are presented. The final stress value for the NMDS ordination was 0.15.
To look for similarities in Fraxinus fungal communities between Far East Russia and Europe, we compared identified fungal taxa on F. mandshurica to recently published literature9,10,29,30,31,32 which report associated fungal taxa to diseased and healthy F. excelsior trees. Of the 20 most frequent fungal OTUs in Far East Russia, 12 were similar to species detected on F. excelsior in Europe (Table 1). Uniquely associated to F. mandshurica were Coniozyma leucospermi, Hannaella coprosmaensis and species of Mycosphaerella, Dioszegia, Paraphoma, and an unknown Helotiales.
Population genetics of H. fraxineus in Far East Russia versus Europe
The European population was represented from 25 isolates, 15 originating from Sweden and 10 from Lithuania, and the Asian population was represented by 10 samples in Far East Russia, 5 from each of two locations (sites 1 and 3, see Fig. 3). No clones were found among the MLGs in either of two populations. The allelic diversity was larger in Asia than in the European population; the total number of alleles found was 75, of which only 8 were shared; 28 alleles were found in the European population and 57 in the Asian population (Table 2). The numbers of missing alleles were low in the European population; only two loci (Chafra03 and mHp_108810) each had one missing value. The numbers were greater in the Asian population, where half of the samples failed to amplify or showed ambiguous results for loci SSR211 and mHp_108810, and between 2 and 4 of the 10 samples did not amplify for loci SSR38, Chafra04, mHp_080497, mHp_080495 and mHp_079915.
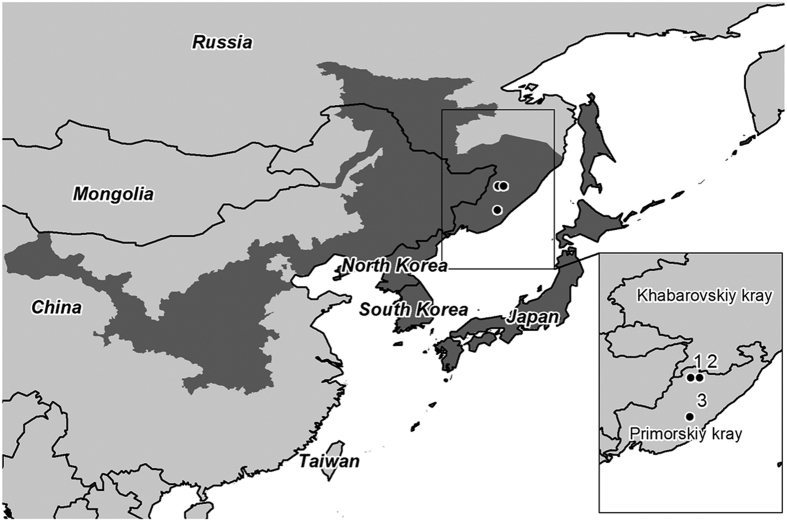
Figure 3. Map showing the approximate natural distribution range of Fraxinus mandshurica in East Asia (shaded area) and the three study sites (closed circles) in Primorye region of Far East Russia.
Source data for mapping distribution of F. mandshurica:18,59,60. Software: ArcGIS version 10.3 (www.esri.com).
Table 2. Primers used in the study, their final primer concentration, annealing temperature and number of alleles shared, fragment sizes found in European and Asian populations respectively.
| Primer namea | Final primer concentration in PCRb | Annealing temperature (°C) | Shared alleles | Only found in European population | Only found in Asian population |
|---|---|---|---|---|---|
| mHp_067022 | 0.2 | 56 | – | 250 | 244, 247, 253 |
| mHp_098984 | 0.4 | 56 | 92 | 98 | 89, 95, 105 |
| mHp_108810 | 0.1 | 56 | – | 264, 268, 277 | 270, 272, 274, 282, 292 |
| mHp_060142 | 0.15 | 56 | – | 164, 170 | 151, 158, 167, 173 |
| mHp_080495 | 0.2 | 56 | 150 | 143 | 146, 148, 152, 156, 160 |
| mHp_080497 | 0.3 | 56 | – | 245, 254 | 248, 251 |
| mHp_079915 | 0.25 | 56 | – | 184, 201 | 175, 181, 187, 194, 204, 222 |
| mHp_095481 | 0.2 | 56 | 138 | 147 | 134 |
| Chafra_03 | 0.2 | 60 | 189 | 208 | 185, 192 |
| Chafra_04 | 0.2 | 60 | – | 101, 111 | 95, 97, 99, 105 109 |
| Chafra_09 | 0.2 | 60 | 134 | 144 | 125, 131, 140 |
| Chafra_13 | 0.2 | 60 | 176, 180 | – | 166, 170, 172, 178, 184 |
| SSR_211 | 0.2 | 64 | 296 | 298 | 292, 294, 314 |
| SSR_38 | 0.2 | 64 | 314 | – | – |
| SSR_58 | 0.2 | 57 | 185 | – | – |
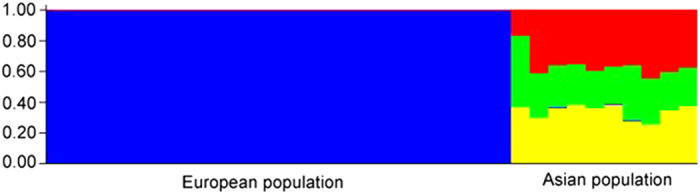
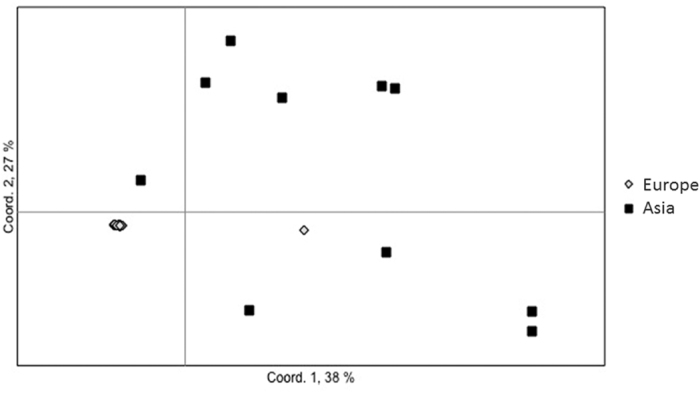
The AMOVA confirmed large differentiation between the two populations, 46% of the molecular variance was found between the European and Asian populations, whereas the remaining 54% was found within the populations (p = 0.0001). The Bayesian clustering analysis of H. fraxineus in Structure was based on all 15 loci. The number of populations (K) showing the highest likelihood was 4 (Fig. 4) and it shows a clear division between the European and Asian populations. To further investigate the population differentiation, a PCoA was produced (Fig. 5). The first axis explained 38% and the second axis 27% of the variation between samples. All European samples cluster close together in one group, except for one sample. The only difference with that particular sample is that it has a missing value for locus mHp_108810, which is probably why it is separated from the other European samples. The Asian samples show much larger variation, a few cluster with the European samples and the others show a large genetic variation.
Figure 4. Population structure in Bayesian clustering program Structure (K = 4) for European (i.e. Swedish and Lithuanian) population versus Asian (i.e. Far East Russian) population.
The three populations from Asia were not separated among the three different sites.
Figure 5. PCoA showing population differences between European and Asian population.
Discussion
In this study, we used high-throughput DNA sequencing to decipher fungal assemblages inhabiting healthy, asymptomatic F. mandshurica leaves. Our results show that individual trees have a highly diverse assemblage (up to 49 distinct fungal taxa detected), though the majority (96%) of OTUs were represented by only 20 taxa, mostly dominated by ascomycetes. Many of the OTUs best matched annotated Gen-Bank accessions of phyllosphere fungi previously detected on plant species other than Fraxinus, though not necessarily from the same geographic region as this study. Rarefaction curves and NMDS analysis revealed little intra-host variability in the phyllosphere fungal assemblages, with several overlapping OTUs, despite the large (up to 250 km) geographic separation between sites.
Recent studies describing associated fungi to the foliage and stems of both healthy and diseased F. excelsior in Europe9,10,29,30,31,32 were useful in comparing fungal profiles to that of F. mandshurica in the East. In many cases similar fungal taxa were detected (Table 1) though few identified at the species level in this study. The most similar taxa included Cladosporium sp., Phomopsis sp., Phoma sp., Alternaria alternata, Cryptococcus foliicola, Diaporthe nobilis, Periconia byssoides, Ramularia sp. and Fusarium sp., which suggests these are more generalist fungal species with a cosmopolitan distribution on a variety of host species.
The ascomycete H. fraxineus has been previously documented in Japan4,16,33, north-eastern China34, and Korea35. Previously H. fraxineus has been reported from Primorsky region, Far East Russia on decaying rachises F. mandshurica4, however this is the first report of its occurrence most notably on healthy, asymptomatic F. mandshurica within its natural distribution range in the East. H. fraxineus was detected on asymptomatic leaves of F. mandshurica at all three sites. Site 3 (Fig. 3) which had a markedly higher frequency of detection on leaves (55%) was also more geographically distant to the other sites. Reasons for the higher level of detection here are not clear, though we cannot exclude the possibility of variable microclimatic conditions among sites that may have influenced both the timing of H. fraxineus fruiting on previous year’s rachises, the stage of development of localized infections in host tissue at the time leaves were sampled, or both. Compared to other fungal taxa, H. fraxineus had much lower number of sequence reads that may be attributable to several factors including 1) possible DNA degradation during the storage and processing of samples, 2) the timing in which samples were collected (e.g. samples collected late in the season or prior to leaf senescence/leaf shed may exhibit a higher detection frequency than that found in the tested material), 3) endophytic colonization of H. fraxineus being restricted to relatively small colonies on the sampled leaves, and 4) antagonistic effects of other more dominant fungi (e.g. Mycosphaerella spp., see below) that may actively limit further growth and establishment of H. fraxineus.
The most dominant OTU in our study could not be identified to the species level, though the closest GenBank record belonged to Mycosphaerella sp. (Table 1). Mycosphaerella species are among the largest genera of plant pathogenic fungi that include more than 3000 species and several thousand anamorphs that lack known teleomorph connections36. Species of Mycosphaerella vary in their ecological role on a wide range of host plants, e.g. as plant pathogens, saprobes, and/or endophytes, though in this study, Mycosphaerella sp. was found to be associated with seemingly healthy leaves. Another fungal taxa associated to F. mandshurica was Coniozyma leucospermi. Ascomycetous fungi with Coniothyrium-like anamorphs are common colonisers of wood and leaves of broadleaved plants. They have also been recognized as biological control agents against plant pathogens37, and producers of secondary metabolites that may function to inhibit activity related to human diseases38. The biological relevance of both of these dominant species to the overall fitness of F. mandshurica, its influence on the community composition structure, and in particular its interactions with H. fraxineus, warrants further investigation.
The habitat of H. fraxineus (syn. Lambertella albida s. Hosoya et al.) has been described as a saprophyte decaying leaves of F. mandshurica var. japonica16,33. This lifestyle behaviour is similar to that of its sister relative H. albidus – a well-known decomposer of F. excelsior leaves with widespread distribution throughout Europe, but now with rather limited occurrence following the introduction of H. fraxineus. In a previous study that characterized the temporal dynamics of the foliar endophytic community of F. excelsior throughout the growing season (May–October, during 2008) using isolation and ITS sequencing, H. albidus was never detected30. This might suggest that H. albidus in fact does not establish endophytic infections in leaves of F. excelsior during its period of active growth in the same manner that H. fraxineus does on F. mandshurica within its native range in the East, or that the fungus has been outcompeted by other fungi such that it was not found.
As we have shown in this study, the presence of H. fraxineus in asymptomatic leaves of F. mandshurica suggests the fungus to possess a biphasic lifestyle, switching from endophytic to saprotrophic life stages on its native host. During its endophytic stage, H. fraxineus probably establishes inconspicuous infections on F. mandshurica leaves that are highly localized and, at least for some period, in a state of quiescence. In this system, the interactions with F. mandshurica may be considered both balanced and antagonistic to explain the apparent symptomless colonization of leaves, in effect resulting in a tolerance of the host to the fungus. Yet it is important to recognize that even balanced antagonistic interactions may be plastic and have the potential for variability and evolutionary development either in the direction of more highly specialized mutualism or parasitism and exploitation39. Furthermore, endophytism does not exclude different life history strategies such as the ability to grow saprophytically on dead or senescing tissues following a period of endophytic growth. As an early colonizer with a prolonged endophytic stage, H. fraxineus effectively establishes an ‘advantage of position’ that follows with rapid colonization of tissues when suitable conditions are met (e.g. onset of senescence)40, and which gives advantage over competing fungi. Innocuous endophytes may also exist as quiescent pathogens41, having an unpredictable period of fungal latency, and only produce symptoms and cause disease when the host is subjected to physiological stress42; a compatible scenario for trees planted outside their natural distribution range.
Understanding the continuum of endophyte to parasitic lifestyle and the mechanisms involved in determining what makes a particular organism pathogenic on one host and not on another, is undeniably complex. Multiple studies have reported shifts in lifestyles from endophytic to pathogenic stages, and back43,44,45. Frequently, endophytes are sister species to virulent pathogens on the same, or closely related, host species46,47. Changed abiotic and biotic conditions or single mutations can cause common symptomless endophytes to switch to active growth and saprobic exploitation of the substrate44,48. A novel host environment may provide little natural resistance to an organism that is flexible in occupying new, yet similar ecological territory – the concept of niche opportunity. Subsequent to this new adaptation, lifestyle changes coincident with the inherent resistance of a new host onto which it successfully ‘jumped’, occurs. Such extreme unprecedented cases, although rare, can result in widespread devastation. A classic example is the Chestnut blight caused by Cryophonectria parasitica, a benign associate of Japanese Chestnut (Castanea crenata) that was introduced to eastern North America in the early 1900’s. In the 50 years that followed its introduction, North American chestnuts (Castanea dentata), which dominated forest ecosystems in eastern North America, were near eliminated.
A more complex issue of concern is the increasing incidence of new invasive pathogens worldwide, our lack of understanding of associated organisms of plants moving through the international plant trade (including lesser known endophytes, and many unknowns), and our ability to predict whether beneficial organisms will have the same mutualistic relationship in a novel niche environment. This is especially relevant for plant quarantines where innocuous phases would not surprisingly be overlooked. Endophytes and pathogens may possess many of the same virulence factors (effectors) necessary to infect and colonize the host to break down leaf components and obtain nutrients to survive as saprotrophs, but these factors are only active in certain life stages. In the case of H. fraxineus, it would have been near to impossible to predict that a benign associate of F. mandshurica, which before recently was not well characterized, would cause the widespread devastation that engulfs the European population of F. excelsior today. Indeed, many forest pathogens now considered invasive in Europe exist in a similar balanced system as having benign associations in their native origin.
In this study we also compared the genetic diversity of the H. fraxineus population in Far East Russia to the newly established population of H. fraxineus in Europe. Most of the microsatellite markers used in the study were developed based on European populations, and proved to be useful also for the Asian samples. Our results confirm those earlier reported17 comparing European and Japanese populations: the genetic diversity is larger in Asia than in Europe, and the pathogen must have gone through a bottleneck, most probably as a result of the species introduction to Europe through a very limited number of individuals.
Fraxinus mandshurica has been introduced to Europe consistently during the previous century for amenity plantings. Reports associating symptomatic F. mandshurica with H. fraxineus in Estonia is rather perplexing since a general lack of symptoms on planted Asian Fraxinus species (including F. mandshurica) has been widely observed in various parks and natural arboretums in Sweden between the years 2011 and 2014 (M. Cleary, unpublished data) and from a variety of Asian Fraxinus species in Denmark23, though the possibility cannot be excluded that some symptoms on F. mandshurica may be attributable to certain provenances of the species being not well suited to the site19, or other predisposing biological factors. The nature of the plasticity of endophytism allows for latent pathogen behaviour to become opportunistic on e.g. physiologically-stressed trees. Purportedly the natural (original) hosts of H. fraxineus are F. mandshurica and F. chinensis since these ash species are the only hosts of H. fraxineus thus far reported from Asia6,34. However, a larger number of Fraxinus species are native to Asia and located near or within the natural distribution range of F. mandshurica that extends from northeast China to the far east of Russia (see distribution map, Fig. 3). Hence, one cannot exclude the possibility that other Asian Fraxinus species also provide a suitable niche for H. fraxineus, and which have also been exported to other countries as nursery stock from those regions. In our observations of exotic Fraxinus planted in Southern Sweden, F. mandshurica, F. chinensis, F. floribunda, F. paxiana, F. platypoda, and F. sieboldiana showed no visible crown damage, while European and many North America Fraxinus species exhibit varying degrees of characteristic symptoms and crown dieback (M. Cleary, unpublished data). Indeed, more information is needed to clarify the extent of the host range for H. fraxineus with other indigineous Fraxinus species originating from Asia.
Since H. fraxineus was pathogenic on artificial stem-wound inoculations on F. mandshurica var. japonica seedlings21 this might suggest that the active mechanisms that determine susceptibility are found in the leaves following the more ‘natural’ course of establishment via spore germination, appressorium formation and penetration of host tissue8. The role of leaf senescence/leaf shed as a mechanism of disease escape in F. excelsior trees in Europe is somewhat ambiguous49,50,51. It is obvious that H. fraxineus could indeed develop freely in woody tissue21, so in theory any inherent traits such as early leaf shed may aid in limiting the establishment of H. fraxineus in the stems. Interestingly, senescence in F. mandshurica in its native environment occurs earlier and more rapidly compared to several other common broadleaved tree species, with all leaves usually shed by early September52, and typically much earlier than F. excelsior throughout most of Europe.
The endophytic lifestyle of H. fraxineus on F. mandshurica, particularly concerning molecular interactions established during penetration, infection and colonization of plant tissue, compared to other hosts such as F. excelsior that have not co-evolved with the fungus, can provide clues for better understanding mutualism and pathogenesis and identify commonalities and disparity in host recognition patterns. Inoculation techniques that mimic natural spore infection of F. excelsior by H. fraxineus using a closed, moist chamber system8 are apt for material studies of host-pathogen interactions under conditions more realistic to natural systems (as opposed to artificial wound-inoculations).
Further studies are also needed on the biology of H. fraxineus in its native environment on indigineous Fraxinus species in Asia to elucidate the time course for sporulation and infection development in leaves and rachises up until leaf senescence and leaf shed. Moreover, the fungal assemblages of eastern Fraxinus species and their interaction with H. fraxineus warrants further investigation. Of particular interest would be to isolate and test the antagonistic capacity of Mycosphaeralla spp. and/or other endophytic fungi uniquely associated to F. mandshurica against H. fraxineus, and their feasibility as potential biological control agents of H. fraxineus on F. excelsior in Europe.
Methods
Sites and sampling of material
During summer 2012, up to 15 similarly sized asymptomatic leaves (including leaflets and rachises) were randomly collected from the crown of three F. mandshurica trees, each growing at three different locations (46°37.148′N, 134°55.545′E; 46°37.319′N, 135°23.0155′E; 44°36.276′N, 134°52.011′E) in mixed forests in the Primorye region, Far East Russia (Fig. 3). Leaves were devoid of any damage symptoms. Leaves were labelled in the field and then dried before storage and processing. In the lab, for each leaf, leaflets and rachises were separated into individual samples, and homogenized in a Lab Wizz micro ball mill (Laarmann, The Netherlands). A total of 75 samples were prepared for sequencing of the fungal community.
DNA extraction, PCR amplification and sequencing
Total DNA was extracted with CTAB buffer with added 2% (w/v) polyvinylpolypyrrolidone. The ITS region of the rDNA was amplified by PCR using the general primers53, gITS7 and ITS4. Each sample was uniquely barcoded. PCR was performed in 50 μl reactions and consisted of the following final concentrations, 0.25 ng μL−1 template DNA, 200 μM of dNTPs; 750 μM of MgCl2; 0.025 μM polymerase (5 U/μL) (DreamTaq Green, Thermo Scientific, Waltham, USA), and 200 nM of each primer in 1× buffer. Amplifications were performed using the Applied Biosystems 2720 thermal cycler. The PCR program started with denaturation at 95 °C for 5 min, followed by 30 cycles of 95 °C for 30 s, annealing at 56 °C for 30 s and 72 °C for 30 s, followed by a final extension step at 72 °C for 7 min. To check if the PCR was successful, the PCR products were visualized by gel electrophoresis on 1% agarose gel stained with Nancy-520 (Sigma-Aldrich, Sweden). PCR products were purified using Agencourt AMPure XP (Agencourt Bioscience Corp, Massachusetts USA) and with the ‘E.Z.N.A. Cycle-Pure’ kit (Omega) following manufacturer’s instructions. After quantification of PCR products using a Qubit flurometer 2.0 (Life Technologies, Sweden), samples were pooled in an equimolar mix and sent for 454-amplicon sequencing using the GS FLX Titanium chemistry (Macrogen Inc, Seoul, Korea).
Confirmation of Fraxinus species
A subsample of extracted DNA from the above samples from each of the three sites was selected for determination of Fraxinus species. The primers trnH_psbA3/trnHf_05, rbaLa/rbcLa, and matK_390/matK_132654,55 were used amplify the nuclear ribosomal intergenic spacer chloroplast regions. Optimal temperature regimes for PCR were established individually by testing amplification success for each primer. Amplifications were performed using the Veriti Thermal Cycler (Applied Biosystems) in 50 μl reactions containing the following final concentrations, 2.5 ng/μl template DNA, 0.025 μM Taq Polymerase (5 U/μL), 200 μM of dNTPs, 750 μM of MgCl2, and 0.2 μM of each primer in 1× Buffer. Cycle conditions were initial denaturation at 94 °C for 4 min, followed by 35 cycles of 94 °C for 30 s, annealing for 30 s at 62 °C for trnH_psbA3/trnHf_05, 60 °C for rbaLa/rbcLa, and 50 °C for matK_390/matK_1326, and 72 °C for 30 s, with a final extension at 72 °C for 7 min. PCR products were verified as above and purified using E.Z.N.A. Cycle-Pure kit (Omega) following manufacturer’s instructions. Prepared samples were sequenced by GS FLX Titanium chemistry (Macrogen Inc, Seoul, Korea).
Sequence analysis
Fungal ITS data derived from 454-amplicon sequencing was processed using the bioinformatics pipeline SCATA available at Department of Forest Mycology and Plant Pathology, Swedish University of Agricultural Sciences (SLU), (http://scata.mykopat.slu.se). Quality filtering removed sequences considered too short (<200 bp), with low mean read quality of <20, and those missing either primers. There were 39,772 sequences that passed the quality control thresholds, and sequences were clustered into operational taxonomic units (OTUs), which we consider to be taxonomically distinct, at 2% dissimilarity. OTUs were identified 1) in the SCATA program by comparing them with reference sequence databases at SLU Department of Forest Mycology and Plant Pathology and UNITE56, and 2) by alignment with blastn at GenBank (NCBI). The distinction between rare species and PCR and sequencing errors cannot be easily done and thus, singletons and doubletons were excluded from the dataset. To determine whether there were differences in the community among the three localities, species rarefaction curves were generated from the 20 most frequently occurring OTUs in the vegan package in R. One curve was generated for each site. Nonmetric Multidimensional Scaling (NMDS) ordination was carried out in the vegan package, using the metaMDS function, specifying the Bray-Curtis dissimilarity index, three dimensions and 1000 iterations. The first two dimensions were used to visualize the relative abundances of the 20 most frequently occurring OTUs across the study. Convex hulls were drawn for samples from each site. Shannon-Wiener diversity indices was used to compare the fungal communities of F. mandshurica trees among the three localities. Sequences obtained from chloroplast primers were aligned and manually edited using Lasergene software package SeqMan Pro (DNA Star, Madison WI, USA). Species identification was confirmed by comparing the acquired sequences to those deposited in GenBank through blastn search.
Microsatellite analysis
Hymenoscyphus fraxineus-positive samples identified via 454-amplicon sequencing were verified via PCR amplification with specific primers, forward 5′-AGC TGG GGA AAC CTG ACT G-3′, and reverse 5′-ACA CCG CAA GGA CCC TAT C-3′57 prior to microsatellite analysis. PCR was performed in 10 μl reaction volumes in a master mix similar to that stated above. The cycling conditions including initial denaturation at 95 °C for 5 min followed by 35 amplification cycles of denaturation at 94 °C for 30 s, annealing at 62 °C for 1 min and extension at 72 °C for 30 s. The reaction was finished by an extension step at 72 °C for 7 min. PCR products were visualized by gel electrophoresis on a 1% agarose gel in SB buffer.
Initially samples were tested using 26 microsatellites (MS) from earlier studies13,14,58 of which 15 amplified fragments in both Asian and European samples were selected for fragment analysis of samples (Table 2). Another set of European H. fraxineus samples comprising 15 cultured isolates from Sweden and 10 cultured isolates from Lithuania were used for comparison. All European isolates were derived from diseased tissues of ash (for isolate locations see Supplementary table15). PCR amplification was performed in 15 μL reaction volume containing the following final concentrations; 0.025 μM of DreamTaq Polymerase (Thermo Scientific), 1× buffer, 0.2 μM of MgCl2, 200 μM of dNTPs, 0.2 or 0.3 μM of each primer (see Table 2) and 1 ng/μl of template DNA. Cycling conditions varied by primer, denaturation for 3 min (5 min for mHp primers) at 95 °C, 35 cycles of 30 s at 95 °C (94 °C for mHp and SSR primers), 30 s at the annealing temperature (see Table 1), 45 s at 72 °C, and a final extension step of 7 min at 72 °C. Successful amplifications were confirmed as previously noted above. Samples were analyzed at SciLife Lab, Uppsala University, Sweden on the ABI 3730XL DNA Analyzer. GeneMarker (Softgenetics) was then used to determine the lengths of the fragments and identify different genotypes at each locus.
Population genetic analysis
If more than one peak was present in the sample, the dominant peak was recorded. If any sample showed ambiguous result, no record was entered for that particular sample and locus. The results were compiled and multilocus genotypes (MLGs) were created for each sample. If any sample had more than five missing values, the sample was discarded from the analysis. To analyze the allele frequencies, genetic distance and analysis of molecular variance (AMOVA), the Excel-add-in software GenAlEx 6.5 was used. To visualize the genetic differences, a principal coordinate analysis (PCoA) was performed based on the genetic distances using the same software. To infer the common ancestry for the different samples, the Bayesian clustering program Structure version 2.3.4 was used. For each run, the first 500 000 iterations were discarded as burn-in, followed by 500 000 iterations for data collection. The populations were assumed to be in Hardy-Weinberg equilibrium and the alleles were assumed to be independent from each other. The number of populations (K) tested was 1–12 and each was replicated three times. To identify the K value, the average LnP(D) of each K was calculated.
Additional Information
How to cite this article: Cleary, M. et al. Friend or foe? Biological and ecological traits of the European ash dieback pathogen Hymenoscyphus fraxineus in its native environment. Sci. Rep. 6, 21895; doi: 10.1038/srep21895 (2016).
Supplementary Material
Acknowledgments
We gratefully acknowledge Dmitrii Shabunin at the Saint Petersburg Research Institute of Forestry for his assistance with the Fraxinus mandshurica material, Eva Wallander for her advice regarding Fraxinus species identification, Katarina Ihrmark for her invaluable support and advice in the lab, Jacob Assmann for technical assistance, and Gintautas Mozgeris at the ASU Faculty of Forest Science and Ecology, Institute of Forest Management and Wood Science in Lithuania for his assistance with GIS spatial tools. This research was supported by Carl Tryggers Stiftlesen (CTS 12:522), The Swedish Research Council FORMAS and EU Cost Action FP1103 FRAXBACK.
Footnotes
Author Contributions M.C. led and wrote the manuscript, performed research on the fungal community using next generation sequencing (NGS); D.N. performed multivariate analyses on the NGS dataset and wrote on the manuscript; D.M. performed laboratory research for NGS of the fungal community and population genetics studies, and contributed to the writing of the manuscript; A.B. analyzed the microsatellite datasets, performed the population genetic study analyses and wrote on the manuscript; R.V. provided theoretical support and made the study possible by acquiring the source material from Far East Russia, and J.S. provided theoretical support and wrote on the manuscript.
References
- Kowalski T. Chalara fraxinea sp. nov. associated with dieback of ash (Fraxinus excelsior) in Poland. Forest Pathol. 36, 264–270 (2006). [Google Scholar]
- Kowalski T. & Holdenrieder O. Pathogenicity of Chalara fraxinea. Forest Pathol. 39, 1–7 (2009). [Google Scholar]
- Queloz V., Grunig C. R., Berndt R., Kowalski T., Sieber T. N. & Holdenrieder O. Cryptic speciation in Hymenoscyphus albidus. Forest Pathol. 41, 133–142 (2011). [Google Scholar]
- Baral H. O. & Bemmann M. Hymenoscyphus fraxineus vs. Hymenoscyphus albidus – A comparative light microscopic study on the causal agent of European ash dieback and related foliicolous stroma-forming species. Mycology. 5, 228–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral H. O., Queloz V. & Hosoya T. Hymenoscyphus fraxineus, the correct scientific name for the fungus causing ash dieback in Europe. IMA Fungus 5, 79–80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A., Holdenrieder O., Pautasso M., Queloz V. & Sieber T. N. Hymenoscyphus pseudoalbidus the causal agent of European ash dieback. Mol. Plant Pathol. 15, 5–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisits T., Kritsch P., Kräutler K., Matlakova M. & Halmschlager E. Ash dieback associated with Hymenoscyphus pseudoalbidus in forest nurseries in Austria. JARED. 4, 230–235 (2012). [Google Scholar]
- Cleary M. R., Daniel G. & Stenlid J. Light and scanning electron microscopy studies of the early infection stages of Hymenoscyphus pseudoalbidus on Fraxinus excelsior. Plant Pathol. 62, 1294–1301 (2013). [Google Scholar]
- Bakys R., Vasaitis R., Barklund P., Ihrmark K. & Stenlid J. Investigations concerning the role of Chalara fraxinea in declining Fraxinus excelsior. Plant Pathol. 58, 284–292 (2009). [Google Scholar]
- Bakys R., Vasaitis R., Barklund P., Thomsen I. M. & Stenlid J. Occurrence and pathogenicity of fungi in necrotic and non-symptomatic shoots of declining common ash (Fraxinus excelsior) in Sweden. Eur. J. For. Res. 128, 51–60 (2009). [Google Scholar]
- Kraj W., Zarek M. & Kowalski T. Genetic variability of Chalara fraxinea, dieback cause of European ash (Fraxinus excelsior L.) Mycol. Progress 11, 37–45 (2012). [Google Scholar]
- Rytkönen A., Lilja A., Drenkhan R., Gaitnieks T. & Hantula J. First record of Chalara fraxinea in Finland and genetic variation among isolates sampled from Aland, mainland Finland, Estonia and Latvia. Forest Pathol. 41, 169–174. (2011). [Google Scholar]
- Bengtsson S. B. K., Vasaitis R., Kirisits T., Solheim H. & Stenlid J. Population structure of Hymenoscyphus pseudoalbidus and its genetic relationship to Hymenoscyphus albidus. Fungal Ecol. 5, 147–153 (2012). [Google Scholar]
- Gross A., Grünig C. R., Queloz V. & Holdenrieder O. A molecular toolkit for population genetic investigations of the ash dieback pathogen Hymenoscyphus pseudoalbidus. Forest Pathol. 42, 252–264 (2012). [Google Scholar]
- Burokiene D. et al. Genetic population structure of the invasive ash dieback pathogen Hymenoscyphus fraxineus in its expanding range. Biol Invasions 10.007/s10530-015-0911-6 (2015). [DOI] [Google Scholar]
- Zhao Y.–J., Hosoya T., Baral H.-O., Hosaka K. & Kakishima M. Hymenoscyphus pseudoalbidus, the correct name for Lambertella albida reported from Japan. MycoTaxon 122, 25–41 (2013). [Google Scholar]
- Gross A., Hosoya T. & Queloz V. Population structure of the invasive forest pathogen Hymenoscyphus pseudoalbidus. Mol Ecol. 23, 2943–2960 (2014). [DOI] [PubMed] [Google Scholar]
- Shabunin D. A., Semakova T. A., Davydenko E. V. & Vasaitis R. A. Ash decline in nature monument ‘Dudergof heights’, caused by the fungus Hymenoscyphus pseudoalbidus, and morphological features of its ascospores. In: Proceedings of the Saint- Petersburg Forest Research Institute. 2012, 70–79 (2012). [Google Scholar]
- Drenkhan R., Adamson K. & Hanso M. Fraxinus sogdiana, a Central Asian ash species is susceptible to Hymenoscyphus fraxineus. Plant Protect. Sci. 51, 150–152 (2015). [Google Scholar]
- Drenkhan R., Sander H. & Hanso M. Introduction of Mandshurian ash (Fraxinus mandshurica Rupr.) to Estonia, Is it related to the current epidemic on European ash (F. excelsior L.)? Eur. J. For. Res. 133, 769–781 (2014). [Google Scholar]
- Gross A. & Holdenrieder O. Pathogenicity of Hymenoscyphus fraxineus and Hymenoscyphus albidus towards Fraxinus mandshurica var. japonica. Forest Pathol. 45, 172–174 (2015). [Google Scholar]
- Drenkhan. R. & Hanso M. New host species for Chalara fraxinea. New Dis. Rep. 22, 16. [ 10.5197/j.2044-0588.2010.022.016] (2010). [DOI] [Google Scholar]
- McKinney L. V., Neilsen L. R., Collinge D. B., Thomsen I. M., Hansen J. K. & Kjær E. J. The ash dieback crisis: genetic variation in resistance can prove a long-term solution. Plant Pathol 63, 485–499 (2014). [Google Scholar]
- Petrini O. Fungal endophytes of tree leaves. In: Microbial Ecology of Leaves (eds Andrews J. & Hirano S. S.). Springer Verlag, New York, pp. 179–197 (1991). [Google Scholar]
- Newton A. C., Fitt B. D. L., Atkins S. D., Walters D. R. & Daniel T. J. Pathogenesis, parasitism and mutualism in the trophic space of microbe-plant interactions. Trends Microbiol. 18, 365–373 (2010). [DOI] [PubMed] [Google Scholar]
- Rodriguez R. & Redman R. More than 400 million years of evolution and plants still can’t make it on their own, plant stress tolerance and habitat expansion via fungal symbiosis. Comparative Biochemistry and Physiology – Part A. Mol. Integr. Physiol. 146, 20 (2007). [Google Scholar]
- Wilkinson H. H., Siegel M. R., Blankenship J. D., Mallory A. C., Bush L. P. & Schardl C. L. Contribution of fungal loline alkaloids to protection from aphids in a grass-endophyte mutualism. MPMI. 13, 1027–1033 (2000). [DOI] [PubMed] [Google Scholar]
- Arnold A. E. et al. Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences of the United States of America 100, 15649–15654 (2003). [DOI] [PMC free article] [PubMed]
- Davydenko K., Vasaitis R., Stenlid J. & Menkis A. Fungi in foliage and shoots of Fraxinus excelsior in eastern Ukraine, a first report on Hymenoscyphus pseudoalbidus Forest Pathol. 43, 462–467 (2013). [Google Scholar]
- Scholtysik A., Unterseher M., Otto P. & Wirth C. Spatio-temporal dynamics of endophyte diversity in the canopy of European ash (Fraxinus excelsior). Mycol Progress. 12, 291–304 (2013). [Google Scholar]
- Hauptman T., Piskur B., de Groot M., Orgis N., Ferlan N. & Jurc D. Temperature effect on Chalara fraxinea, heat treatment of saplings as possible disease control method. Forest Pathol. 43, 360–370 (2013). [Google Scholar]
- Kowalski T. & Czekaj A. Disease symptoms and fungi on dying ash trees (Fraxinus excelsior L.) in Staszów Forest District stands. Leśne Prace Badawcze (Forest Research Papers) 71, 357-368 [in Polish with English summary] (2010).
- Hosoya T., Otani Y. & Furuya K. Materials for the fungus flora of Japan (46). Trans Mycol Soc Japan. 34, 429–432 (1993). [Google Scholar]
- Zheng H.-D. & Zhuang W.-Y. Hymenoscyphus albidoides sp. nov. and H. pseudoalbidus from China. Mycol. Progress. 10.1007/s11557-013-0945-z (2014). [DOI] [Google Scholar]
- Han J.-G., Shrestha B., Hosoya T., Lee K.-H., Sung G.-H. & Shin H.-D. First report of the ash dieback pathogen Hymensocyphus fraxineus in Korea. Mycobiol. 42, 391–396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aptroot A. Mycosphaerella and its Anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5, Utrecht, The Netherlands (2006).
- Finch-Savage W. E., Clay H. A., Budge S. P., Dent K. C., Clarkson J. P. & Whipps J. M. Biological control of Sclerotinia pseudotuberosa and other fungi during moist storage of Quercus robur seeds. Eur. J. Plant Pathol. 109, 615–624 (2003). [Google Scholar]
- Damm U., Verkley G. J. M., Crous P. W., Fourie P. H., Haegi A. & Riccioni L. Novel Paraconiothyrium species on stone fruit trees and other woody hosts. Persoonia 20, 9–17 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B. & Boyle C. The endophytic continuum. Mycol Res. 109, 661–686 (2005). [DOI] [PubMed] [Google Scholar]
- Sieber T. N. (2007) Endophytic fungi in forest trees, are they mutualists? Fungal Biol. Rev. 21, 75–89 (2005). [Google Scholar]
- Stone J. K., Bacon C. W. & White J. E. Jr. An overview of endophytic microbes: Endophytism defined. In: Microbial Endophytes (eds) Bacon C. W., White Jr J. F., Marcel, Dekker Inc., New York, New York, USA, pp 3–29 (2000). [Google Scholar]
- Brown A. V. & Macaskill G. Shoot diseases of pine. Forest Research Information Note 69. Forestry Commission, Edinburgh. (2005).
- Hyde K. D. & Soytong K. The fungal endophyte dilemma. Fungal Div. 33, 163–173 (2008). [Google Scholar]
- Eaton C. J., Cox M. P. & Scott B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 180, 190–195 (2011). [DOI] [PubMed] [Google Scholar]
- Freeman S. & Rodriguez R. J. Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science 260, 75–78 (1993). [DOI] [PubMed] [Google Scholar]
- Andrew M., Baru R., Short S. M. & Kohn L. M. Evidence for a common toolbox based on necrotrophy in a fungal lineage spanning necrotrophs, biotrophs, endophytes, host generalists and specialists. PlosOne 7(1), e29943, 10.1371/journal.pone.0029943 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaye L., Garciá-Guzmán G. & Heil M. Endophytes versus biotrophic and necrotrophic pathogens—are fungal lifestyles evolutionarily stable traits? Fungal Div. 60, 125–135 (2013). [Google Scholar]
- Álvarez-Loayze P. et al. Light Converts Endosymbiotic Fungus to Pathogen, Influencing Seedling Survival and Niche-Space Filling of a Common Tropical Tree, Iriartea deltoidea. PlosOne 6(1), e16386, 10.1371/journal.pone.0016386 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney L. V., Nielsen L. R., Hansen J. K. & Kjær E. D. Presence of natural genetic resistance in Fraxinus excelsior (Oleraceae) to Chalara fraxinea (Ascomycota), an emerging infectious disease. Heredity 106, 788–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stener L.-G. Clonal differences in susceptibility to the dieback of Fraxinus excelsior in southern Sweden. SJFR. 28, 205–16 (2013). [Google Scholar]
- Kirisit s. T. & Freinschlag C. Ash dieback caused by Hymenoscyphus pseudoalbidus in a seed plantation of Fraxinus excelsior in Austria. JARED 4, 184–191 (2012). [Google Scholar]
- Koike T., Kitao M., Maruyama Y., Mori S. & Lei T. T. Leaf morphology and photosynthetic adjustments among deciduous broad-leaved trees within the vertical canopy profile. Tree Physiol. 21, 951–958 (2001). [DOI] [PubMed] [Google Scholar]
- Ihrmark K. et al. New primers to amplify the fungal ITS2 region – evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol. 82, 666–677 (2012). [DOI] [PubMed] [Google Scholar]
- Pang X. et al. Utility of the trnH–psbA Intergenic Spacer Region and Its Combinations as Plant DNA Barcodes: A Meta-Analysis. PlosOne. 10.1371/journal.poe.0048833 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallander E. Systematics of Fraxinus (Oleaceae) and evolution of dioecy. Plant Syst. Evol. 273, 25–49 (2008). [Google Scholar]
- Abarenkov K. et al. The UNITE database for molecular identification of fungi – recent updates and future perspectives. New Phytol. 286, 281–285 (2010). [DOI] [PubMed] [Google Scholar]
- Johansson S. B. K., Vasaitis R., Ihrmark K., Barkland P. & Stenlid J. Detection of Chalara fraxinea from tissue of Fraxinus excelsior using species-specific ITS primers. Forest Pathol. 40, 111–115 (2010). [Google Scholar]
- Gherghel F. et al. The development of a species-specific test to detect Hymenoschyphus pseudoalbidus in ash tissues. Forest Pathol. 44, 137–144 (2014). [Google Scholar]
- Fund W. Manchurian mixed forests. (2014). Available at: http://www.eoearth.org/view/article/154434 (accessed February 22, 2015).
- Yihong W. Study of the ecology of Fraxinus mandshurica. Journal of Northeast Forestry University 6, 61–64 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.