Abstract
Introduction
Only a third of obese patients develop chronic ventilatory failure. This cross-sectional study assessed multiple factors potentially associated with chronic ventilatory failure.
Materials/patients and methods
Participants had a body mass index (BMI) >30 kg/m2, with or without chronic ventilatory failure (awake arterial partial pressure of carbon dioxide >6 kPa or base excess (BE) ≥2 mmols/L). Factors investigated were grouped into domains: (1) obesity measures, (2) pulmonary function, (3) respiratory and non-respiratory muscle strength, (4) sleep study derivatives, (5) hypoxic and hypercapnic responses, and (6) some hormonal, nutritional and inflammatory measures.
Results
71 obese participants (52% male) were studied over 27 months, 52 (SD 9) years and BMI 47 (range 32–74) kg/m2. The best univariate correlates of BE from each domain were: (1) dual-energy X-ray absorptiometry measurement of visceral fat (r=+0.50, p=0.001); (2) supine forced expiratory volume in 1 s (r=−0.40, p=0.001); (3) sniff maximum pressure (r=−0.28, p=0.02); (4) mean overnight arterial oxygen saturation (r=−0.50, p<0.001); (5) ventilatory response to 15% O2 breathing (r=−0.28, p=0.02); and (6) vitamin D (r=−0.30, p=0.01). In multivariate analysis, only visceral fat and ventilatory response to hypoxia remained significant.
Conclusions
We have confirmed that in the obese, BMI is a poor correlate of chronic ventilatory failure, and the best independent correlates are visceral fat and hypoxic ventilatory response.
Trial registration number
Keywords: Sleep apnoea, Respiratory Muscles, Non invasive ventilation
Key messages.
It remains unclear why only some obese patients develop hypoventilation syndrome.
In the largest cross-sectional study to date, exploring a wide range of potential factors provoking the obesity hypoventilation syndrome, we have found that intra-abdominal obesity and a poor response to hypoxia appear to be the dominant factors associated with hypoventilation, thus providing further insights into the pathophysiology of this condition.
The best independent correlates are visceral fat and hypoxic ventilatory response?
Introduction
Patients with obesity-related chronic ventilatory failure have higher morbidity, mortality and healthcare utilisation, compared with eucapnic obese participants.1 The clinical features of obesity (body mass index, BMI >30 kg/m2), and daytime hypercapnia (arterial partial pressure of carbon dioxide, PaCO2 >6 kPa), in the absence of any other discernible cause, define obesity hypoventilation syndrome (OHS), while some also include the presence of sleep-disordered breathing (SDB) in the definition.2 Only a third of patients with obesity develop chronic ventilatory failure,3 and the reasons for this are poorly understood. We have prospectively measured a wide range of factors potentially impacting on ventilatory failure in a cross-sectional study of obese participants, with and without chronic ventilatory failure, in order to rank the possible key respiratory and non-respiratory factors associated with the presence of chronic ventilatory failure.
Methods
Study design and setting
This was an open, cross-sectional study of obese participants, with and without chronic ventilatory failure, carried out between June 2011 and September 2013 in the Oxford Sleep Unit, Churchill Hospital, UK. An earlier article based on this group of patients, demonstrating that an isolated raised base excess (BE) is part of the spectrum of OHS, has already been published.4 Further details of the methods can be found in the online supplementary data. The study was approved by the Oxford Research Ethics Committee (Oxfordshire REC B 11/H0605/9) and pre-registered (ClinicalTrials.gov Identifier: NCT01380418).
Participants
A random sample of obese participants (BMI >30 kg/m2) with or without chronic ventilatory failure were recruited following referral to the sleep and ventilation clinic, or during assessment for possible bariatric surgery. Participants were excluded if: currently on drugs potentially stimulating/depressing respiration, diuretics, or theophylline; had obstructive lung disease (forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio <70% predicted); had other severe comorbities, for example, congestive cardiac failure, primary central nervous system or neuromuscular diseases, and untreated hypothyroidism; or were currently on treatment with continuous positive airways pressure or non-invasive ventilation. No patients were recruited who had a respiratory illness during the previous 4 weeks.
In addition to basic and demographic information, six potential respiratory and non-respiratory mechanistic domains that could contribute to chronic ventilatory failure were assessed: obesity and its distribution; lung function; respiratory and non-respiratory muscle strength; sleep-related measures; ventilatory response; and hormonal, nutritional and inflammatory measures.
Basic and demographic data
Age, sex, height, weight, BMI, resting arterial oxygen saturation (SpO2), and daytime somnolence (Epworth Sleepiness Score (ESS)5) were measured. Venous blood and radial arterial blood (breathing air) were sampled with participants in a seated position between 8:00 and 10:00, after at least 15 min rest. Oxygen tension (arterial partial pressure of oxygen, PaO2), carbon dioxide tension (PaCO2), pH, standard bicarbonate concentration and BE were measured on an analyser maintained and calibrated according to the manufacturer's recommendations (ABL 90, Radiometer, West Sussex, UK).
Measures of obesity and its distribution
Body fat and its distribution were assessed in several ways: waist (cm), hip (cm), neck (cm), dual-energy X-ray absorptiometry (DXA, including the estimated visceral adipose tissue component in the android region; Lunar, GE Healthcare, Chalfont St Giles, UK), and bioimpedance (Bodystat 1500 analyser, Bodystat Ltd, Douglas, Isle of Man, UK). An estimation of visceral adipose tissue by DXA was only available from participants with a BMI ≤40; the algorithm estimating visceral fat, which essentially subtracts cutaneous fat from the overall abdominal fat, has not been verified beyond this level of obesity.6 7
Lung function
Lung function was measured sitting and supine: FEV1, FVC, FEV1/FVC ratio, relaxed vital capacity and expiratory reserve volume.
Muscle strength
Respiratory muscle strength was assessed seated from pressures (cm H2O) at the mouth: maximum inspiratory pressure (MIP) after maximal expiration at residual volume, maximal expiratory pressure after maximal inspiration, at total lung capacity (MicroRPM, CareFusion, Basingstoke, UK); and sniff nasal inspiratory pressures at functional residual capacity, during five maximal sniffs, in a standardised manner as previously described.8 Non-respiratory muscle strength was also assessed.
Sleep-related measures
SDB was assessed from a one-night, in-hospital, respiratory polygraphic sleep study (Win-Visi monitoring system; Stowood Scientific Instruments, Oxford, UK).
Ventilatory responses
Two-point ventilatory responses were measured to hypoxia (15% O2, poikilocapnic) and hypercapnia (5% CO2, balance O2).
Hormonal, nutritional and inflammatory measures
Several hormonal, nutritional and inflammatory variables were measured, potentially related to muscle weakness and poor diet: fasting glucose and insulin, glycated haemoglobin, vitamin D, brain natriuretic peptide, iron studies, vitamin B12, folate, fatty acids, thyroid-stimulating hormone, leptin, adiponectin and C reactive protein. Fasting blood samples were taken in the morning following the overnight sleep study.
Further information on some of the methods is contained in the online supplementary data.
Statistical methods
Data analyses were performed using SPSS (V.22, IBM Corporation Ltd, USA). Data were tested for normality and presented as the mean and SD unless otherwise indicated. Statistical analysis for the potential correlates of ventilatory failure was a two-stage process. First, within each mechanistic domain, Pearson's analysis was used to find the measures that most correlated with the degree of ventilatory failure (using both arterial CO2 and BE). Then the best independent representatives of each mechanistic domain, most likely to be causal rather than simply reflecting the degree of ventilatory failure, were entered into a final forward stepwise multiple regression analysis to identify the overall ranking of independent correlates of ventilatory failure. All data were collected from all patients apart from the quadriceps muscle strength, which was performed on only the last 40 patients due to late availability of the equipment. The measurement of visceral adipose tissue by DXA was only available from participants with a BMI ≤40, as the algorithm has not been verified beyond this level of obesity.
Results
Seventy-one obese participants (52% male) were studied. Forty-one obese participants were referred from the bariatric service (60% female; BMI 48.1) and 30 participants from the sleep and ventilation clinic (55% male; BMI 45.3). Table 1 shows the arterial blood gas data.
Table 1.
Arterial blood gases and acid–base balance
| Variable | Mean | SD | Range (0– 100%) |
|---|---|---|---|
| pH | 7.42 | 0.03 | 7.35 to 7.48 |
| PaO2 (kPa) | 9.95 | 1.21 | 6.55 to 12.10 |
| PaCO2 (kPa) | 5.57 | 0.80 | 4.16 to 9.63 |
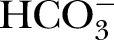 (mmol/L) (mmol/L) |
26.2 | 2.11 | 21.50 to 33.30 |
| Base excess (mmol/L) | 2.08 | 2.41 | −3.5 to +10 |
Mean (SD) values for arterial blood gas measurement for the whole group, with range (0–100%).
PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of carbon dioxide.
Individual domain analyses
Measures of obesity and its distribution
Table 2 shows the stronger correlations between chronic ventilatory failure (BE and PaCO2) and age, and the measures of obesity and its distribution. BMI correlated poorly with chronic ventilatory failure defined either by PaCO2 or BE (r=+0.21, p=NS; r=+0.20, p=NS, respectively). The strongest correlation was with the DXA measurements of visceral fat (BE, r=+0.50; p=0.001), although these were only available from the 43 participants with a BMI ≤40 kg/m2. Neck circumference and the impedance measure were significantly correlated with either PaCO2 or BE (r=−0.28, p=0.02, r=−0.27, p=0.03, respectively), albeit the relationships were weaker. There were no other correlates between the measures of obesity and chronic ventilatory failure.
Table 2.
Age, and obesity indices
| Variable | Mean | SD | Range (0–100%) | Correlation to BE (p value) | Correlation to PaCO2 (p value) |
|---|---|---|---|---|---|
| Age (years) | 52.0 | 8.9 | 26–74 | +0.06 (0.63) | +0.17 (0.17) |
| Weight (kg) | 136.3 | 29.5 | 77.8–229 | +0.19 (0.11) | +0.18 (0.13) |
| BMI (kg/m2) | 47.2 | 9.8 | 32.3–73.9 | +0.20 (0.09) | +0.21 (0.08) |
| Neck (cm) | 46.5 | 4.8 | 38–58 | +0.15 (0.22) | +0.28 (0.02) |
| Waist (cm) | 134.8 | 20.9 | 102–180 | +0.21 (0.08) | +0.22 (0.07) |
| Hip (cm) | 133.9 | 20.2 | 101–177 | +0.18 (0.13) | +0.18 (0.15) |
| Impedance (ohms) | 382.3 | 86.9 | 194–704 | −0.18 (0.15) | −0.27 (0.03) |
| Visceral adipose tissue mass (kg) | 3.42 | 1.17 | 1.31–5.64 | +0.50 (0.001) | +0.36 (0.02) |
Mean (SD) for variables in the ‘distribution of fat’ domain, with range (0–100%). The obesity measures shown are those exhibiting the highest correlations with BE and PaCO2 (p values in brackets). There were no other significant correlations between obesity measures and either BE or PaCO2. Note, visceral adipose tissue estimates from DXA were only available for participants with BMI ≤40, n=43.
BE, base excess; BMI, body mass index; DXA, dual-energy X-ray absorptiometry; PaCO2, arterial partial pressure of carbon dioxide.
Lung function
Online supplementary table S1 shows the stronger correlations between ventilatory failure and measures of lung function. Supine FEV1 had the strongest correlation with the BE (r=−0.40, p<0.001), although all the standard spirometric measures, seated or supine, were correlated with both BE and PaCO2.
Muscle strength
Online supplementary table S2 shows the stronger correlations between ventilatory failure and measures of muscle strength. The maximum sniff pressure was the strongest correlate of the BE (r=−0.28, p=0.02), although the MIP approached significance (r=−0.22, p=0.06). There were no other significant correlations with non-respiratory muscle strength measures.
Sleep-related measures
Online supplementary table S3 shows the stronger correlations between chronic ventilatory failure and the sleep study derivatives. The measures of overall nocturnal hypoxia (mean overnight %SaO2, and the %time below 90% SaO2) were the strongest correlates of chronic ventilatory failure (BE, r=−0.50, p<0.001 and r=+0.46, p<0.001, respectively). The oxygen desaturation index (ODI) was also correlated (BE, r=+0.34, p=0.004), but there was no correlation with the apnoea hypopnoea index.
Hypoxic and hypercapnic ventilatory response
Online supplementary tables S4 and S5 show the stronger correlations between chronic ventilatory failure and the baseline ventilation measurements, the ventilatory response to breathing 15% oxygen and to 5% CO2 in oxygen. The poikilocapnic ventilatory response to hypoxia was significantly correlated with the BE (r=−0.28, p=0.02). The fall in SaO2 during 15% O2 breathing was actually the most correlated with BE (r=+0.46, p<0.001), but part of this measure will depend on the baseline PaCO2 and PaO2 themselves.
Hormonal, nutritional and inflammatory measures
Online supplementary table S6 shows the stronger correlations between ventilatory failure and various hormonal, nutritional and inflammatory measures. Vitamin D levels inversely correlated with BE (r=−0.3, p=0.01) and were generally low, with 19% having levels which are classified as severely deficient (<12.5 nmol/L).9 10 There were only two other significant correlates: BNP and transferrin. Iron in serum is mainly transported by transferrin.11 All but one BNP level (102 ng/mL) were below the usual threshold to define heart failure (100 ng/L).
Regression analysis across all the respiratory and non-respiratory domains
The upper part of table 3 shows the strongest significant independent correlates from each of the individual respiratory and non-respiratory domains that could be potentially causal. The lower part of table 3 shows the result of the regression analysis of all these six factors. The only two significant factors associated with chronic ventilatory failure were the DXA measure of intra-abdominal (visceral) fat, and the poikilocapnic ventilatory response to breathing 15% oxygen. Vitamin D levels were the next most significant, but only reached a significance level of 0.19. All the other factors were not significant in the final model.
Table 3.
Linear regression analysis using the best independent correlates of BE from each domain
| Correlation coefficient | p Value | |
|---|---|---|
| Best independent correlates from each domain | ||
| Obesity | ||
| Visceral adipose tissue volume by DXA scan | +0.50 | 0.001 |
| Lung function | ||
| FEV1%, supine | −0.40 | <0.001 |
| Sleep variables | ||
| Mean overnight oxygen saturation | −0.50 | <0.001 |
| Ventilatory control | ||
| Ventilatory response to 15% oxygen | −0.28 | 0.02 |
| Respiratory muscle strength | ||
| Sniff maximum (cm H2O) | −0.28 | 0.02 |
| Hormonal, nutritional and inflammatory measures | ||
| Vitamin D | −0.30 | 0.01 |
| Cumulative correlation coefficient | p Value | |
| Significant independent correlates | ||
| Visceral adipose tissue volume by DXA scan | +0.49 | 0.004 |
| Ventilatory response to 15% oxygen | +0.58 | 0.04 |
Results for the best correlates of BE in a linear regression for each individual domain. The correlate was chosen on the basis of the likelihood of it having a causal association with BE (eg, ventilatory response to 15% oxygen was chosen over the absolute fall in SaO2, and vitamin D was chosen over both BNP and transferrin). The lower part of the table shows the result for the best overall significant independent correlates of BE in a multiple linear regression containing the best correlate from each domain. A cumulative correlation coefficient of 0.58 indicates that the model accounts for 34% of the variance in BE. Vitamin D levels were the next most significant correlate, but with a p value of 0.19.
BE, base excess; BNP, brain natriuretic peptide; DXA, dual-energy X-ray absorptiometry; FEV1, forced expiratory volume in 1 s.
Discussion
In this prospective, hypothesis-generating, observational cross-sectional study of a group of obese individuals, we have confirmed that BMI alone is a relatively poor correlate of ventilatory failure. Age was also not a significant correlate, implying it is not simply that it takes time for a given level of obesity to provoke ventilatory failure. There are a number of strong correlations in each of the biological domains, suggesting that ventilatory failure in obesity is likely to be multifactorial. However, the main independent correlations were the presence of intra-abdominal fat on DXA scan, and a reduction in the ventilatory response to 15% oxygen.
It might be argued that, due to the shape of the haemoglobin dissociation curve, the ventilatory response, and the absolute fall in SaO2, during 15% O2 breathing would be expected to be greater if initially more hypoxic, and therefore already on the steeper part of the curve. This would be true to some extent for the absolute SaO2 fall during 15% oxygen breathing. However, while it is accepted that the physiological response to hypoxia is dependent on numerous factors, the ventilatory response to hypoxia has been shown to be rendered linear when plotted against SaO2, thus the slope of the ventilatory response to hypoxia (ΔV/Δ%SaO2) should not depend on where one starts on the dissociation curve.12 Furthermore, the change in minute ventilation during the 15% O2 challenge test was also correlated with the BE, which would not be expected if the reduced ventilatory response was simply the result of the initial position on the dissociation curve.
Other studies have also shown that patients with OHS have a reduced ventilatory response to hypoxia;13 14 failure to maintain ventilation in the face of nocturnal hypoventilation is a plausible contributory cause to the development of diurnal hypoventilation. The extra nocturnal hypoventilation14 seen in these obese individuals is presumably provoked by some other factor, with the reduced hypoxic ventilatory response playing a permissive role. Removal of carotid bodies in humans and animals reduces hypoxic drive and leads to a gradual development of chronic ventilatory failure.15 Repeated periods of extranocturnal hypoventilation will lead to renal bicarbonate retention that may not be fully cleared during waking hours, leading to a gradual increase over time, with the alkalosis-suppressing awake ventilation. This was the explanation advanced by Berger et al,16 and modelled by Norman et al.17
Alternatively, the chronic hypoxia of OHS may have blunted the ventilatory response to hypoxaemia. There is inconsistent evidence for a reduced ventilatory drive to hypoxia following, for example, long-term exposure to altitude in lowlanders.18–21 However, even if acquired blunting is the explanation in our patients, it would still imply that restoration of ventilatory drive should be considered as a therapeutic target.
Finally, it may be that the blunted hypoxic response in our patients was simply due to the mechanical fat load, although we think this unlikely for three reasons: those with eucapnia, but an isolated bicarbonate retention, had been able to return their PaCO2 to normal during the day, yet still exhibited a reduced hypoxic ventilatory response,4 there were patients in our cross-sectional with normal lung volumes and muscle strength who exhibited reduced hypoxic response, but normal hypercapnic responses; and there was very little correlation between hypoxic and hypercapnic responses (r=0.08, p=0.53), which one would have expected if they were dominantly impaired by pump overload.
Preferential abdominal obesity is a plausible contributory cause to the gradual development of daytime hypercapnia. A cross-sectional population-based study including nearly 122 000 participants showed that abdominal obesity was the strongest correlate of impaired lung function21 and early work in this area identified a reduced expiratory reserve volume as a possible provoker of obesity-related hypoventilation.22 Sutherland et al,23 using a wide range of body fat variables (including DXA to determine the effect of fat distribution on lung volumes), found in healthy adults that lung volumes were only loosely associated with BMI. Variables derived from both DXA and non-DXA measurements, reflecting upper body fat, had highly significant negative correlations with functional residual capacity and expiratory reserve volume in both men and women.
Our findings, albeit within the BMI range 30–40, suggest that more fat, actually inside the abdominal cavity, may be especially important. Particularly on lying down, the weight of abdominal fat will restrict the descent of the diaphragm; this will also narrow small airways with an increase in airflow resistance and intrinsic positive end expiratory pressure.24 The diaphragm may even be overstretched, reducing muscle fibre contraction efficiency.25 This effect of a raised intra-abdominal pressure on ventilation is well recognised;26 27 in individuals with other pathologies that raise intra-abdominal pressure (particularly seen in acutely ill patients in intensive care), additional obesity is a significant extra risk factor for ventilatory failure.28 It may be that intra-abdominal fat is more significant than subcutaneous abdominal wall fat at embarrassing the diaphragm when abdominal compartment expansion is limited, and thus effectively under pressure. However, more data are required at higher obesity levels over a BMI of 40.
An interesting observation in our data set was that neck circumference was a better correlator of DXA visceral fat (r=+0.66, p<0.001) than either waist circumference (r=+0.27, p=0.10) or waist/hip ratio (r=+0.19, p=0.24). This underlines the problem that obstructive sleep apnoea (OSA) may indirectly code for the metabolic syndrome in cross-sectional studies seeking to explore the relationship between OSA and cardiovascular consequences.
Interpreting the sleep data correlates of ventilatory failure as possible evidence for any primary causal role is also difficult. Lower nocturnal saturations may reflect combinations of an initially lower starting SaO2, nocturnal hypoventilation secondary to abdominal fat loading, poor hypoxic drive permissive of yet further hypoventilation, all of which will lead eventually to bicarbonate retention and diurnal ventilatory failure. There was no suggestion within these data that the presence of OSA (measured either as the apnoea/hypopnoea index or ODI) was contributory.
Nearly 20% of the patients in our study were severely deficient in vitamin D, possibly contributing to a low-grade proximal myopathy;29 thus, a pilot trial of vitamin D replacement in those deficient might be appropriate. However, vitamin D levels did not significantly correlate with our measures of either ventilatory or non-ventilatory muscle strength. The explanation for higher transferrin levels correlating with worse ventilatory failure is not clear. Higher transferrin levels would normally suggest iron deficiency, but no other iron status measure corroborated this. Isolated raised transferrin can occur with raised oestrogen levels30 that are also found in the obese.31
None of our patients had clinically detectable heart failure, and this was confirmed by the essentially normal BNP levels. The strong correlation between BNP levels (albeit within the normal range) and the markers of ventilatory failure is likely to be a result rather than a cause. Reduced oxygen tensions are an independent factor regulating the synthesis and release of natriuretic peptides, and hypoxia response elements have also been found in the promoter sequence of the human BNP gene.32
A significant limitation to this study was that the estimation of visceral adipose tissue by DXA was not available from participants with a BMI >40, as the relevant algorithms have not been verified beyond this level of obesity.33 Thus, it is possible that this relationship might not continue to be true for BMI values above 40. However, it is encouraging that the relationship did exist, even within the relatively narrow range of BMI values (30–40).
Our work suggests that reversing the gradual accumulation of bicarbonate may be an alternative strategy in managing obesity hypoventilation. This has not been considered safe in most other examples of hypercapnic ventilatory failure, where the respiratory pump is failing or overwhelmed, such as neuromuscular disease or severe chronic obstructive lung disease, when increasing ventilatory drive is likely to be ineffective and potentially dangerous. Thus, it would seem reasonable to perform a randomised controlled trial of acetazolamide, or equivalent, in patients with obesity hypoventilation, who are not in acute decompensation, despite this not being currently recommended.34 There are already small uncontrolled trials of acetazolamide in obesity looking at hypercapnic drive and nocturnal hypoxia that are suggestive of improvement in patients with central sleep apnoea.35
Acknowledgments
Many thanks and appreciation to Richard Madgewick for his help with lung function and ventilatory drive measurements and Debby Nichols and Barbara Winter for their help with the sleep measurements of the study.
Footnotes
Funding: This work was supported by the Oxford Health Services Research Committee, the Oxford NIHR Biomedical Research Centre, and the Oxford Radcliffe Hospital Charitable Funds. The NICO 2 device was kindly donated by Philips-Respironics, UK.
Competing interests: None declared.
Ethics approval: Oxfordshire REC B 11/H0605/9.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The authors are happy for the raw data to be available to readers.
References
- 1.Nowbar S, Burkart KM, Gonzales R et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004;116:1–7. doi:10.1016/j.amjmed.2003.08.022 [DOI] [PubMed] [Google Scholar]
- 2.Berger KI, Ayappa I, Chatr-Amontri B et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001;120:1231–8. doi:10.1378/chest.120.4.1231 [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Jones RL, Man GC. Hypercapnic ventilatory response in patients with and without obstructive sleep apnea: do age, gender, obesity, and daytime PaCO(2) matter? Chest 2000;117:454–9. doi:10.1378/chest.117.2.454 [DOI] [PubMed] [Google Scholar]
- 4.Manuel AR, Hart N, Stradling JR. Is a raised bicarbonate, without hypercapnia, part of the physiological spectrum of obesity-related hypoventilation? Chest 2015;147:362–8. doi:10.1378/chest.14-1279 [DOI] [PubMed] [Google Scholar]
- 5.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- 6.Clasey JL, Bouchard C, Teates CD et al. The use of anthropometric and dual-energy X-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res 1999;7:256–64. doi:10.1002/j.1550-8528.1999.tb00404.x [DOI] [PubMed] [Google Scholar]
- 7.Kaul S, Rothney MP, Peters DM et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012;20:1313–18. doi:10.1038/oby.2011.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JM, Moxham J, Green M. The maximal sniff in the assessment of diaphragm function in man. Clin Sci 1985;69:91–6. doi:10.1042/cs0690091 [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Binkley NC, Bischoff-Ferrari HA et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. doi:10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 10.Valcour A, Blocki F, Hawkins DM et al. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab 2012;97:3989–95. doi:10.1210/jc.2012-2276 [DOI] [PubMed] [Google Scholar]
- 11.Rouault TA, Klausner RD. Iron-sulfur clusters as biosensors of oxidants and iron. Trends Biochem Sci 1996;21:174–7. doi:10.1016/S0968-0004(96)10024-4 [PubMed] [Google Scholar]
- 12.Rebuck AS, Campbell EJ. A clinical method for assessing the ventilatory response to hypoxia. Am Rev Respir Dis 1974;109:345–50. doi:10.1164/arrd.1974.109.3.345 [DOI] [PubMed] [Google Scholar]
- 13.Han F, Chen E, Wei H et al. Treatment effects on carbon dioxide retention in patients with obstructive sleep apnea-hypopnea syndrome. Chest 2001;119:1814–19. doi:10.1378/chest.119.6.1814 [DOI] [PubMed] [Google Scholar]
- 14.Zwillich CW, Sutton FD, Pierson DJ et al. Decreased hypoxic ventilatory drive in the obesity-hypoventilation syndrome. Am J Med 1975;59:343–8. doi:10.1016/0002-9343(75)90392-7 [DOI] [PubMed] [Google Scholar]
- 15.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 2010;90:675–754. doi:10.1152/physrev.00012.2009 [DOI] [PubMed] [Google Scholar]
- 16.Berger KI, Goldring RM, Rapoport DM. Obesity hypoventilation syndrome. Semin Respir Crit Care Med 2009;30:253–61. doi:10.1055/s-0029-1222439 [DOI] [PubMed] [Google Scholar]
- 17.Norman RG, Goldring RM, Clain JM et al. Transition from acute to chronic hypercapnia in patients with periodic breathing: predictions from a computer model. J Appl Physiol 2006;100:1733–41. doi:10.1152/japplphysiol.00502.2005 [DOI] [PubMed] [Google Scholar]
- 18.Joseph V, Pequignot JM. Breathing at high altitude. Cell Mol Life Sci 2009;66:3565–73. doi:10.1007/s00018-009-0143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weil JV, Cherniack NS, Dempsey JA et al. NHLBI workshop summary. Respiratory disorders of sleep. Pathophysiology, clinical implications, and therapeutic approaches. Am Rev Respir Dis 1987;136:755–61. doi:10.1164/ajrccm/136.3.755 [DOI] [PubMed] [Google Scholar]
- 20.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia-mediated plasticity of acute O2 sensing requires altered red-ox regulation by HIF-1 and HIF-2. Ann N Y Acad Sci 2009;1177:162–8. doi:10.1111/j.1749-6632.2009.05034.x [DOI] [PubMed] [Google Scholar]
- 21.Leone N, Courbon D, Thomas F et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009;179:509–16. doi:10.1164/rccm.200807-1195OC [DOI] [PubMed] [Google Scholar]
- 22.Cullen JH, Formel PF. The respiratory defects in extreme obesity. Am J Med 1962;32:525–31. doi:10.1016/0002-9343(62)90053-0 [Google Scholar]
- 23.Sutherland TJ, Goulding A, Grant AM et al. The effect of adiposity measured by dual-energy X-ray absorptiometry on lung function. Eur Respir J 2008;32:85–91. doi:10.1183/09031936.00112407 [DOI] [PubMed] [Google Scholar]
- 24.Steier J, Jolley CJ, Seymour J et al. Neural respiratory drive in obesity. Thorax 2009;64:719–25. doi:10.1136/thx.2008.109728 [DOI] [PubMed] [Google Scholar]
- 25.Sharp JT, Druz WS, Kondragunta VR. Diaphragmatic responses to body position changes in obese patients with obstructive sleep apnea. Am Rev Respir Dis 1986;133:32–7. doi:10.1164/arrd.1986.133.1.32 [DOI] [PubMed] [Google Scholar]
- 26.Hedenstierna G, Larsson A. Influence of abdominal pressure on respiratory and abdominal organ function. Curr Opin Crit Care 2012;18:80–5. doi:10.1097/MCC.0b013e32834e7c3a [DOI] [PubMed] [Google Scholar]
- 27.Sugerman HJ. Effects of increased intra-abdominal pressure in severe obesity. Surg Clin North Am 2001;81:1063–75, vi doi:10.1016/S0039-6109(05)70184-5 [DOI] [PubMed] [Google Scholar]
- 28.Holodinsky JK, Roberts DJ, Ball CG et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013;17:R249 doi:10.1186/cc13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr 2002;75:611–15. [DOI] [PubMed] [Google Scholar]
- 30.Horne CH, Mallinson AC, Ferguson J et al. Effects of oestrogen and progestogen on serum levels of alpha(2)-macroglobulin, transferrin, albumin, and IgG. J Clin Pathol 1971;24:464–6. doi:10.1136/jcp.24.5.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anty R, Dahman M, Iannelli A et al. Bariatric surgery can correct iron depletion in morbidly obese women: a link with chronic inflammation. Obes Surg 2008;18:709–14. doi:10.1007/s11695-007-9276-y [DOI] [PubMed] [Google Scholar]
- 32.Arjamaa O, Nikinmaa M. Hypoxia regulates the natriuretic peptide system. Int J Physiol Pathophysiol Pharmacol 2011;3:191–201. [PMC free article] [PubMed] [Google Scholar]
- 33.Micklesfield LK, Goedecke JH, Punyanitya M et al. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity 2012;20:1109–14. doi:10.1038/oby.2011.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selim BJ, Junna MR, Morgenthaler TI. Therapy for sleep hypoventilation and central apnea syndromes. Curr Treat Options Neurol 2012;14:427–37. doi:10.1007/s11940-012-0188-3 [DOI] [PubMed] [Google Scholar]
- 35.DeBacker WA, Verbraecken J, Willemen M et al. Central apnea index decreases after prolonged treatment with acetazolamide. Am J Respir Crit Care Med 1995;151:87–91. doi:10.1164/ajrccm.151.1.7812578 [DOI] [PubMed] [Google Scholar]


