Abstract
Objectives
The aim of this study was to evaluate the effect of using UVA-induced crosslinking with or without riboflavin as photosensitizers on degradation of dentin matrix by dentin proteases.
Methods
Demineralized dentin specimens (0.4×3×6mm, n=10/group) were subjected to: (RP1), 0.1% riboflavin-5 phosphate/UVA for 1 min; (RP5), 0.1% riboflavin-5 phosphate/UVA for 5 min; (R1), 0.1% riboflavin/UVA for 1 min; (R5), 0.1% riboflavin-UVA for 5 min; (UV1), UVA for 1 min; (UV5), UVA for 5 min. Specimens were incubated in 1 mL zinc and calcium containing media for 1 day and 1 week. An untreated group served as control (CM). After incubation, the loss of dry mass of samples was measured and aliquots of media were analyzed for the release of C-terminal fragment telopeptide (ICTP vs CTX) of collagen to evaluate for cathepsin K (CA-K) and total matrix metalloproteinase (MMP)-mediated degradation. Data were analyzed using repeated measures ANOVA at α=0.05.
Results
Although UVA radiation alone reduced dentin degradation, UVA-activated riboflavin or riboflavin-5 phosphate inhibited MMP and CA-K activities more than UVA alone. The effects of crosslinking were more pronounced in 7-day samples; only with CA-K were the effects of crosslinking with or without photosensitizer significantly different from controls in 1-day samples.
Significance
The use of bioactive forms (RP) or longer treatment time did not result with better effect. The use of UVA crosslinking reduces dentin matrix degradation, especially with photosensitizers.
Keywords: collagen matrices, dentin, crosslinking, riboflavin
1. Introduction
In spite of recent improvements in adhesive dentistry, the durability of resin-dentin bonds is still far from optimal due to enzymatic degradation of collagen fibrils in the hybrid layer by endogenous dentin proteases [1]. Among these enzymes, several matrix metalloproteinases (MMPs) and cysteine cathepsins (CCs) participating on type I collagen degradation have been reported in both intact [2–9] and carious dentin [9–12]. These endogenous proteases are responsible for the host-derived degradation of poorly resin-infiltrated dentin matrix, resulting with the loss of bond strength over time [1]. MMPs are capable of hydrolysis of non-resin infiltrated dentin collagen at neutral pH, whereas cathepsin-K (CA-K: the only cathepsin capable of degrading type I helical collagen) is mainly active under acidic conditions (i.e.pH 5–6) and can also activate MMPs [13]. Phosphoric acid etching of dentin or etching with acidic monomers in resin adhesives leads to activation of both of the classes of proteases [14,15].
Improvements in the stiffness of collagen and the inhibition of proteases are two main strategies to protect the integrity of collagen matrix [13,16]. The effective inhibition of proteases may require targeting both MMPs and CA-K. Crosslinking of dentin collagen improves the stability of bonds by strengthening collagen matrix and by preventing collagen degradation. Recent studies have shown that even brief crosslinker pretreatments are capable of inhibiting endogenous MMP-related dentin degradation [17,18] over time [19].
Ultraviolet A induced crosslinking, a new photo-oxidative method using riboflavins has been reported to improve the mechanical properties of organic dentin matrix [18]. Likewise chemical crosslinking, using Ultraviolet A (UVA) radiation is capable of creating covalent intermolecular crosslinking of collagen fibrils [20, 21] by the release of free oxygen radicals that can break weak intermolecular bonds to generate intermolecular covalent bonds [20–22]. Riboflavin is a biocompatible photosensitizer that can be used to form free radicals when activated by UVA with optimum absorption peaks at 270, 366, 445 nm wavelengths. UVA-induced riboflavin irradiation releases free oxygen radicals that react with collagen amine groups to generate covalent bonds [21]. To be maximally effective, riboflavin in the body fluids should be converted to the phosphorylated bioactive form called riboflavin 5'-phospate (RP) due to the poor solubility of riboflavin in water. However, for local application, there is no need to use the bioactive form, which is more expensive. Additionally, the required riboflavin concentration for photo-activated crosslinking of dentin is similar to its water solubility (0.1–0.5%) [22].
Previously, this photo-reductive cross-linking has been used in ophthalmology to treat keratoconus by using UVA-irradiated riboflavin to enhance the stiffness of corneal collagen fibrils [23–25]. Although previous studies have indicated that the treatment of riboflavin by UVA improves the micromechanical properties of dentin and hybrid layers [18], there are few studies focusing specifically to the inhibition effect of crosslinking on dentin MMPs proteases [17,18], and no information is available of the effect on cathepsins. Also, the effect of radiation time and modification of UVA-induced crosslinking with or without riboflavin on degradation of collagen matrix by MMPs and CCs has not been studied. The aim of this study was to evaluate the effect of using UVA-induced crosslinking with or without different riboflavin concentrations, on degradation of dentin matrix by dentin proteases. The null hypotheses were that: (1) treatment of demineralized dentin by UVA with or without riboflavin would not decrease the degradation of collagen matrix by neither CCs nor MMPs over time, and (2) the effect of UVA-induced crosslinking would depend on application time and concentration.
1. Materials and Methods
1.1. Preparation of Dentin Specimens
Seventy extracted sound human third molars removed during a normal treatment under a protocol with patient’s informed consent from University of Oulu Hospital and Health Care Center were used in this study. The teeth were stored at 4°C in 0.9% NaCl supplemented with 0.02% sodium azide to prevent bacterial growth, and were used within three months after extraction. The enamel and superficial dentin were removed parallel to the occlusal surface by using a low-speed diamond saw (Buehler, Lake Bluff, IL, USA) under water-cooling. Dentin specimens (0.4×3×6mm) were sectioned from the mid-coronal dentin. Mineralized dentin specimens were completely demineralized in 10% H3PO4 for 24 hrs under constant stirring, rinsed in distilled water for 2 hrs and dried in a vacuum desiccator containing anhydrous silica beads for 72 hrs to assess their initial dry mass. Specimens were divided into 7 groups (n=10). Groups were (1) R1, pretreated with 0.1% riboflavin (Sigma, St. Louis, MO, USA) under UVA light for 1 min; (2) R5, pretreated with 0.1% riboflavin under UVA for 5 min; (3) RP1, pretreated with 0.1% riboflavin-5 phosphate (Fluka, St. Louis, MO, USA) under UVA for 1 min; (4) RP5, pretreated with 0.1% riboflavin-5 phosphate under UVA for 5 min; (5) UV1, exposed UVA light alone for 1 min; (6) UV5, exposed UVA light alone for 5 min. Solutions were prepared in water and kept in light-proof test tubes to avoid any light-activation of riboflavin. The demineralized samples were immersed in 200 µl of 0.1% riboflavin or riboflavin-5-phosphate and exposed to UVA for 1 or 5 min under a UV lamp (Philips, Hamburg, Germany; λ = 370 nm at 3 mW/cm2). Demineralized dentin beams were exposed to UVA light at a distance of 1 cm [24]. They were then turned over and the dose was repeated. UVA has been reported to only penetrate 200 µm into 400 µm thick specimens. Thus, each half of the beam would only receive 1 min or 5 min exposures. Untreated demineralized beams served as control (CM). Groups were incubated in individual labeled polypropylene tubes with calcium and zinc containing incubation media containing 5 mM HEPES, 2.5 mM CaCl2·H2O, 0.02 mM ZnCl2, and 0.3 mM NaN3, pH:7.4) [26] for 1 and 7 days at 37°C in a shaker-water bath (60 cycles/min).
1.2. Measurement of Dry Mass Loss Overtime
The dry mass for each dentin specimens individually was measured with an analytical microbalance (XP6 Microbalance, Mettler Toledo, Hightstown, NJ, USA) to assess the hydrolysis of total organic matrix of dentin over time. Following demineralization, samples were transferred to individually labelled 96-well plates and placed in the desiccator for 72 hrs. The initial measurement of dry mass was used as baseline weight of samples. The loss of dry mass was calculated according to the baseline as a percentage after each (1 and 7 days) incubation period. After each measurement, beams were rehydrated for 1h in distilled water at 4°C and placed in corresponding polypropylene tubes containing 0.5 ml media. Specimens were rinsed free of salts for 24 hrs in distilled water at 4°C after incubation.
1.3. Assessment of Released Collagen Telopeptide Fragments
To evaluate the specific role of MMPs and CCs in the type I collagen degradation, release of different C-telopeptide fragments was analyzed in the media. MMP activity releases the crosslinked carboxyterminal telopeptide of type I collagen, so-called ICTP, while CA-K among CCs releases a shorter CTX (C-terminal telopeptide of type I collagen) [27]. To analyse the fragments released during incubations of demineralized dentin by proteases, commercial ELISA kits for CTX (Crosslaps ELISA; Immuno Diagnostics System, Denmark) and ICTP (UniQ EIA, Orion Diagnostica, Finland) were used. 50 µl aliquots of media from each beam were used to quantitate solubilized collagen fragments for each time-point. The measurement was performed for aliquots of 10 dentin beams in a spectrometer (Synergy HT, BioTek Inst. Inc., Vermont, USA) at 450 nm absorbance. Collagen degradation as ICTP and CTX telopeptide fragments was calculated in each assay with a standard curve constructed using standards provided by manufacturer.
1.4. Statistical analysis
The percentage of dry mass loss after incubation was compared to baseline dry mass for each sample individually before incubation. The loss of dry mass and the quantity of ICTP and CTX release in terms of MMPs and CA-K activity were tested for normality (Kolmogorov-Smirnov test). Since the data were normally distributed, data were analyzed by using repeated measures 2-way ANOVA (IBM SPSS v.22, NY, USA and post hoc multiple comparison were tested with Tukey HSD tests (SPSS, IBM Inc. USA). P values of 0.05 were considered to indicate statistical significance.
2. Results
2.1. Loss of Dry Mass
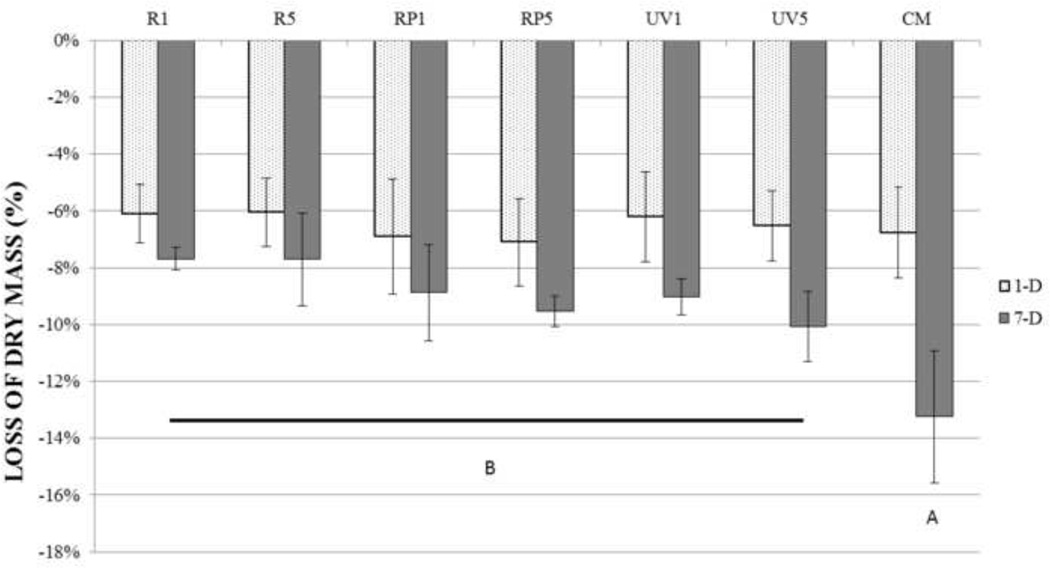
Figure 1 shows the loss of dry mass of demineralized dentin beams at 1 and 7 days. On the far right, the untreated samples (CM) showed a 1 day loss of −6.8±1.6 % dry mass that slowed to a loss of dry mass of −13.2±2.3% after 7 days. In the experimental groups, loss of dry mass at 1 day was not significantly different (p>0.05) than the loss of dry mass in 1- day control. However, after 7 days, all of the experimental groups had lower losses of dry mass than the day 7 of control (p<0.05). Among the experimental groups, loss of dry mass was not significantly different (p>0.05) at 7 days.
Figure 1.
Mean percent loss of dry mass of demineralized dentin beams after 1-day and 7-day incubation was shown. Values are means and standard deviations of the percentage dry mass loss (n = 10). 1-day samples did not show statistically significant differences (p>0.05): the 7-day groups identified by same letter are not significantly different (p > 0.05).
2.2. Release of Collagen Telopeptide Fragments
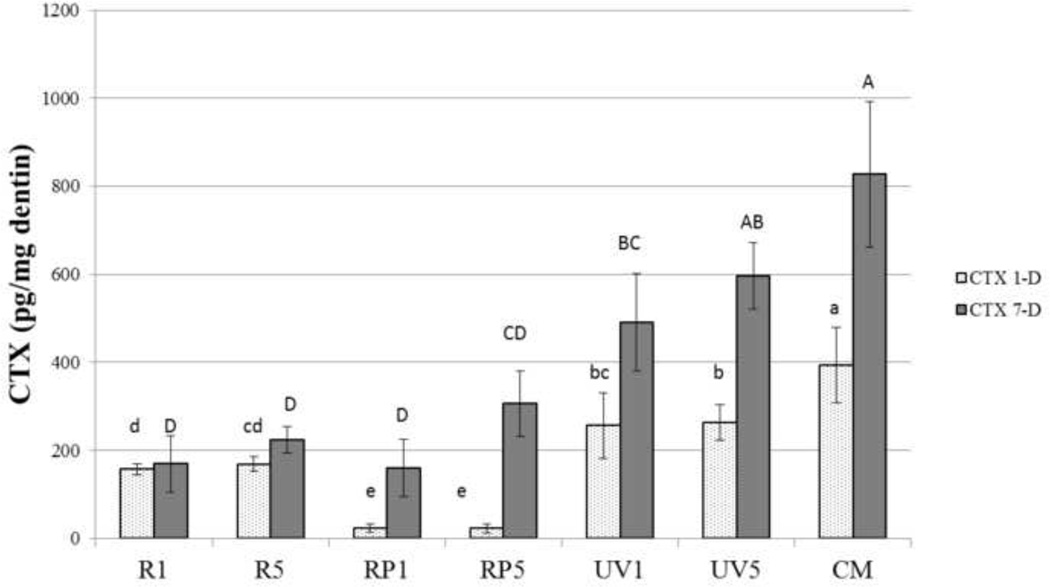
Figure 2 shows the release of the telopeptide CTX produced by CA-K activity. After 1 day of incubation, the untreated control beams released 393±85.9 pg CTX/mg dentin. Treatment of the beams with UVA light for 1 or 5 min significantly reduced the CTX release (p<0.05) to 250±75 pg CTX and 260±75 pg CTX/mg dentin at one day, respectively (Fig. 2). Treatment of demineralized beams with 0.1 % riboflavin with UVA irradiation for 1 or 5 min (R1 and R5) lowered the release of CTX to 169.7±63.8 and 306.7±74.3 pg CTX/mg dentin-1 day, respectively. These values were significantly lower than those produced by 1 or 5 min of UVA irradiation only (UV1 and UV5). The lowest one-day release of CTX was seen in specimens treated with 0.1 % riboflavin 5’-phosphate and irradiated for 1 or 5 min with UVA (RP1 and RP5). These values were 1.1± 0.9 and 3.4±0.6 ng CTX/mg dentin and were significantly lower than all other treatment groups. However, when the fresh media was sampled after 7 days of incubation, the CTX release in the riboflavin 5’-phosphate groups increased significantly (p<0.05) to 169.7±63.8 and 306.7±74.3 pg CTX/mg dry dentin-7 days, respectively. The value for riboflavin 5’-phosphate + UVA light for 1 min (RP1) was 7 times higher than the 1 day value. The 7-day value for riboflavin 5’-phosphate + UVA for 5 min (RP5) was 12 times higher than the 1-day value (Fig. 2).
Figure 2.
The rate of released CTX fragment of various treatment groups was shown after incubation. Values are pg telopeptide/mg dentin for each incubation period (1-day and 7-day). Bar heights are mean values (n = 10); brackets indicate ± SD. Groups with different letters are statistically significantly different (lower case letters for 1-day groups, capital letters for 7-day groups). Statistical analysis was performed with repeated measures ANOVA test to indicate interactions between the groups and incubation period factors “incubation period” (1-day and 7- day) was used as the repeated factor to analyze the data (p < 0.05).
Treatment of demineralized dentin beams with riboflavin + UVA for 1 min but irradiated for 1 vs. 5 min (R1 and R5) produced a 1 day release of CTX of 153±25 and 155±50 pg CTX/ mg dry dentin-1 day. After 7 days, the rate of release was the same, indicating that no more CTX had been released over the next 7 days.
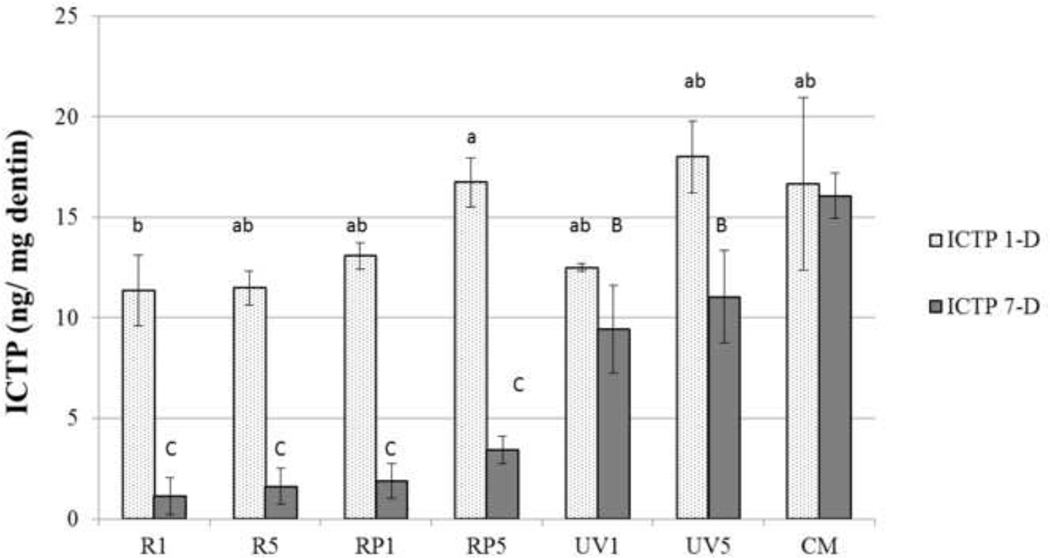
Figure 3 summarizes the release of the telopeptide ICTP by the endogenous MMPs of dentin. The untreated control beams released 16.6±4.1 ng ICTP/mg dry dentin-1 day and 15.6±1.1 ng ICTP/mg dry dentin-7 days (p>0.05). It is unclear why untreated control beams decreased the release of ICTP telopepide fragments after 1 day.
Figure 3.
The result of ICTP liberated from demineralized dentin beams was shown after incubation in SBF. Values are ng telopeptide/mg dentin for each incubation period (1-day and 7-day). Bar heights are mean values (n = 10); brackets indicate ± SD. Statistically significant differences are indicated as in Figure 2.
Treatment of dentin beams with UVA for 1 or 5 min (UV1 and UV5) without riboflavin photosensitizers produced no significant reduction in the release of ICTP telopeptide fragments after 1 day (p>0.05). However, significant reductions in ICTP release (p<0.05) were seen after 7 days (Fig. 3) when compared to the untreated controls was observed.
When UVA treatment was combined with riboflavin 5’-phosphate + UVA (RP1 or RP5) there was no significant reduction in ICTP release in 1 day, but by day 7, the ICTP release was significantly reduced (p<0.05). Treatment of beams with 0.1 % riboflavin + UVA light for 1 or 5 min (R1) produced small but significant (p<0.05) reductions in ICTP release at day 1. Large reductions at day 7 were seen for both R1 and R5 groups (p<0.05).
3. Discussion
The loss of dry mass is a simple, quantitative method for measuring the overall proteolytic degradation of demineralized dentin matrices [19,28,29]. In the current experiment, the loss of dry mass was measured after 1 day and 7 days. The 7 day values represent the cumulative solubilization of insoluble type I collagen, since 90% of the mass of demineralized dentin is due to the presence of insoluble type I collagen [30]
The results of the loss of dry mass indicates that the test null hypothesis that “UV-crosslinking with or without riboflavin does not decrease degradation of collagen by MMPs or CA-K” must be rejected. Although the loss of dry mass seen in experimental groups did not differ at day 1, all treatment groups produced less loss of dry mass after 7 days. This may be due to the fact that there may be two pools of telopeptides. The soluble pool of telopeptides at the matrix surface is already cleaved from collagen telopeptides and is diffusing from the demineralized dentin beam into the medium. The second potential pool of telopeptides are those still attached to the insoluble collagen by pyridinium cross-links that keep the telopeptides tethered to insoluble collagen fibrils.
If UVA-riboflavin cross-linking agents inactivate MMPs and CA-K, the telopeptides already cleaved will continue to diffuse into the medium, but there will be no further cleavage and release of telopeptides. In the latter case, the rates of release of telopeptides at day 1 and day 7 should be similar.
When the media of the control beams (not-cross-linked) were analyzed for CTX release, the 7 day values were about twice that of the 1 day values, rather than 7 times higher, indicating that telopeptide activity falls over time. When beams were irradiated with UVA for 1 and 5 min, the amount of release of CTX telopeptides fell slightly but significantly (p<0.05). This indicated that UVA irradiation alone, without riboflavin, can crosslink and inactivate some, but not all of CA-K activity. The fact that more CTX was released at 7 days compared to 1 day indicates that residual CA-K activity remained after UVA treatment alone.
However, after treatment with 0.1 % riboflavin + UVA irradiation for 1 or 5 min, the amount of release of CTX fell significantly (p<0.05), more than it did following UVA irradiation alone. This suggests that riboflavin enhances the absorption of UVA energy and that it may have completely inactivated CA-K activity. This conclusion is supported by the fact that there was no more CTX release after 1 day. That is, the 1 day and 7 day CTX release were not significantly different.
When riboflavin 5’-phosphate was used to treat the dentin beams, instead of riboflavin alone, the day 1 release of CTX fell to near zero. We speculate that riboflavin 5’-phosphate may have become bound to CTX telopeptides and that the CTX-riboflavin 5’-phosphate complex became bound to CA-K, interfering with its activity. This result may have been reversible, since, at 7 days, CTX was again released into the medium. Future experiments should explore riboflavin vs. riboflavin 5’-phosphate binding to dentin matrices with and without UVA irradiation.
It should be noted that the release of CTX is expressed in pg/mg dry mass, instead of ng/mg dry mass, as the release of ICTP telopeptide fragments were expressed (compare Figs. 2 vs. 3). Only CA-K can release CTX telopeptides from collagen [31]. The optimum pH for CA-K activity is pH 5.0–6.0, but in the current study, the release of CTX was measured at pH 7.4, two pH units above its pH optimum. At pH 7.4, CA-K activity is only 10–11% of its pH 5.5 value [32]. Although CA-K is both a telopeptidase that can release CTX fragments, it is also a powerful collagenase that can cleave helical collagen in more sites than MMP collagenases [33].
When untreated control beams were incubated in calcium and zinc containing media (CM) for 1 or 7 days, the ICTP release was not significantly different (p>0.05). This indicates that although the release of ICTP telopeptides is 20-fold greater than CTX, the high telopeptidase activity of MMPs rapidly depletes the matrix of substrate. When beams were treated with UVA irradiation for 1 or 5 min alone, there was no change in the release of ICTP at 1 day, but there was a significant (p<0.05) reduction in the rate of ICTP release at 7 days (Fig. 3). Thus MMPs seem to be less sensitive to UVA irradiation than is CA-K. However, when demineralized beams were treated with riboflavin or riboflavin 5’-phosphate + UVA irradiation for 1 or 5 min, the release of ICTP did not change after 1 day, but fell significantly (p<0.05) after 7 days. One interpretation of this result assumes that the rate of diffusional efflux of ICTP telopeptide fragments from dentin beams is much slower than the rate of efflux of smaller CTX telopeptide fragments. ICTP telopeptides have molecular masses of 10,249 Da [34], compared to CTX that is <3000 Da [35]. Thus, the diffusion of ICTP from dentin matrices may be slow relative to smaller CTX fragments. When dentin beams were treated with riboflavin plus UVA irradiation, although MMPs may have been crosslinked or inactivated by such treatment, the pool of soluble ICTP telopeptides may have taken many hours to diffuse out of the matrix even though no more ICTP was released by the inactivated MMPs. We speculate that by 7 days, all of that pool of cleaved, soluble ICTP telopeptides had diffused out of the beams, causing the medium ICTP levels at day 7 to be very low since no more ICTP fragments had been cleaved by the inactivated MMPs.
Others have used UV light at 365 nm at 3200 mW/cm2 to strengthen mineralized dentin after irradiation for 5–60 min [36]. UV light exposure for 5–15 min increased the flexural strength of mineralized dentin, while flexural strength fell if dentin was irradiated for more than 30 min probably caused by denaturation of collagen [36]. As those authors used mineralized dentin beams while we used demineralized beams, it is likely that UV energy passed 200 µm into each side of demineralized dentin.
Riboflavins served as photosensitizers to UV light. This may have caused some crosslinking of collagen. Collagen crosslinking is known to make collagen more resistant to bacterial collagenases [36–38]. Thus it is possible that riboflavin-UVA irradiation increased the resistance of dentin collagen to endogenous proteases. We speculate that riboflavin-UVA crosslinking of dentin matrices may also crosslink proteoglycans to collagen [39]. Because glycosaminoglycans are essential for CA-K activity, crosslinking of glycosaminoglycans may inactivate Cat-K. However, there are no published reports of direct inactivation of CA-K/ glycosaminoglycan complexes by UVA –Riboflavin crosslinking.
4. Conclusion
The results of this study indicate that riboflavin crosslinked dentin matrices used with UVA irradiation for 1 to 5 min was enough to block the action of endogenous proteases of dentin. Further long-term studies should be done to determine if the inactivation is permanent. More work should be done to clarify the mechanisms of action of riboflavin-UVA crosslinking in demineralized dentin.
Highlights.
For the first time, we evaluated the inhibitory effect of UVA-induced crosslinking on both MMPs and cathepsin-K.
UVA-induced crosslinking was tested in the presence or absence of photosensitizers (riboflavin and riboflavin-5’-phosphate) in terms of the loss of dry dentin mass and degradation product of MMPs (ICTP) and cathepsin K (CTX).
The results of the study showed that UVA-induced crosslinking significantly inactivated endogenous proteases of dentin.
Acknowledgments
This study was supported by grant #8126472 from the Academy of Finland to AT-M (PI) and by R01 DE015306 from the NIDCR to DHP, P.I.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have a financial interest in products, equipment, and companies cited in the manuscript.
References
- 1.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, et al. Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 2013;29(1):116–135. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Arch Oral Biol. 2000;45(9):757–765. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 3.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. Journal of Dental Research. 2007;86(5):436–440. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 4.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52(2):121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Boukpessi T, Menashi S, Camoin L, Tencate JM, Goldberg M, Chaussain-Miller C. The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials. 2008;29(33):4367–4373. doi: 10.1016/j.biomaterials.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Santos J, Carrilho MR, Tervahartiala T, Sorsa T, Breschi L, Mazzoni A, Pashley D, Tay F, Ferraz C, Tjäderhane L. Determination of matrix metalloproteinases in human radicular dentin. J Endod. 2009;35(5):686–689. doi: 10.1016/j.joen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Mazzoni A, Papa V, Nato F, Carrilho M, Tjäderhane L, Ruggeri A, Jr, Gobbi P, Mazzotti G, Tay FR, Pashley DH, Breschi L. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J Dent. 2011;39(3):231–237. doi: 10.1016/j.jdent.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, Carrilho MR, Pashley DH, Tay FR, Salo T, Tjäderhane L. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36(3):475–481. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Vidal CMP, Tjäderhane L, Scaffa PM, Tersariol IL, Pashley D, Nader HB, Nascimento FD, Carrilho MR. Abundance of MMPs and cysteine cathepsins in caries-affected dentin. J Dent Res. 2014;93(3):269–274. doi: 10.1177/0022034513516979. [DOI] [PubMed] [Google Scholar]
- 10.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77(8):1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 11.Toledano M, Nieto-Aguilar R, Osorio R, Campos A, Osorio E, Tay FR, Alaminos M. Differential expression of matrix metalloproteinase-2 in human coronal and radicular sound and carious dentine. J Dent. 2010;38(8):635–640. doi: 10.1016/j.jdent.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, Nader HB, Salo T, Tjäderhane L, Tersariol IL. Cysteine cathepsins in human carious dentin. J Dent Res. 2011;90(4):506–511. doi: 10.1177/0022034510391906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho MR, Carvalho RM, Tay FR, Pashley DH. Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent Mater. 2013;29(10):999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomater. 2006;27(25):4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114(2):160–166. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90(8):953–968. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, Breschi L, Vallittu P, Tay FR, Pashley DH. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. J Dent Res. 2012;91(2):192–196. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cova A, Breschi L, Nato F, Ruggeri AJ, Carrilho M, Tjäderhane L, Prati C, Di Lenarda R, Tay FR, Pashley DH, Mazzoni A. Effect of UVA-activated riboflavin on dentin bonding. J Dent Res. 2011;90(12):1439–1445. doi: 10.1177/0022034511423397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzoni A, Angeloni V, Apolonio FM, Scotti N, Tjäderhane L, Tezvergil-Mutluay A, Di Lenarda R, Tay FR, Pashley DH, Breschi L. Effect of carbodiimide (EDC) on the bond stability of etch-and-rinse adhesive systems. Dent Mater. 2013;29(10):1040–1047. doi: 10.1016/j.dental.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Cooper DR, Davidson RJ. The effect of ultraviolet irradiation on collagen-fold formation. Biochem J. 1966;98(3):655–661. doi: 10.1042/bj0980655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32(2):279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 22.Tirella A, Liberto T, Ahluwalia A. Riboflavin and collagen: New crosslinking methods to tailor the stiffness of hydrogels. Mater Lett. 2012;74:58–61. [Google Scholar]
- 23.Abbas CA, Sibirny AA. Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers. Microbiol Mol Biol Rev. 2011;75(2):321–360. doi: 10.1128/MMBR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 25.Snibson GR. Collagen cross-linking: a new treatment paradigm in corneal disease – a review. Clin Experiment Ophthalmol. 2010;38(2):141–153. doi: 10.1111/j.1442-9071.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 26.Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, Nishitani Y, Carvalho RM, Looney S, Tay FR, Pashley DH. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent Mater. 2010;26(11):1059–1067. doi: 10.1016/j.dental.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, Qvist P, Delmas PD, Foged NT, Delaissé JM. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res. 2003;18(5):859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 28.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater. 2009;90(1):373–380. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90(4):535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler WT. Dentin collagen: chemical structure and role in mineralization. In: Linde A, editor. Dentin and dentinogenesis. II. Boca Raton: CRC Press; 1984. p. 40. [Google Scholar]
- 31.Karsdal MA, Woodworth T, Henriksen K, Maksymowych WP, Genant H, Vergnaud P, Christiansen C, Schubert T, Qvist P, Schett G, Platt A, Bay-Jensen AC. Biochemical markers of ongoing joint damage in rheumatoid arthritis - current and future applications, limitations and opportunities. Arthritis Res Ther. 2011;13(2):215. doi: 10.1186/ar3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kometani M, Nonomura K, Tomoo T, Niwa S. Hurdles in the drug discovery of cathepsin K inhibitors. Curr Top Med Chem. 2010;10(7):733–744. doi: 10.2174/156802610791113478. [DOI] [PubMed] [Google Scholar]
- 33.Garnero P, Boral O, Byrjalsen I, Ferrara M, Drake FH, Mc Queney MS, Foged NT, Delmas PD, Delaissé JM. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273(48):32347–32352. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 34.Eriksen HA, Sharp CA, Robins SP, Sussi ML, Risteli L, Risteli J. Differently cross-linked and uncross-linked carboxy-terminal telopeptides of type I collagen in human mineralised bone. Bone. 2004;34(4):720–727. doi: 10.1016/j.bone.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Rosenquist C, Fledelius C, Christgau S, Pedersen BJ, Bonde M, Qvist P, Christiansen C. Serum CrossLaps One Step ELISA. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin Chem. 1998;44(11):2281–2289. [PubMed] [Google Scholar]
- 36.Hayashi M, Okamura K, Koychev EV, Furuya Y, Sugeta A, Ota T, Ebisu S. Effects of rehydration on dentin strengthened by heating or UV irradiation. J Dent Res. 2010;89(2):154–158. doi: 10.1177/0022034509354564. [DOI] [PubMed] [Google Scholar]
- 37.Kligman LH, Gebre M. Biochemical changes in hairless mouse skin collagen after chronic exposure to ultraviolet-A radiation. Photochem Photobiol. 1991;54(2):233–237. doi: 10.1111/j.1751-1097.1991.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 38.Nagase H, Visse R. Triple Helicase Activity and the Structural Basis of Collagenolysis Biology of Extracellular Matrix. 2011;2:95–122. [Google Scholar]
- 39.Zhang Y1, Conrad AH, Conrad GW. Effects of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking. J Biol Chem. 2011;286(15):13011–13022. doi: 10.1074/jbc.M110.169813. [DOI] [PMC free article] [PubMed] [Google Scholar]