Abstract
Aim:
The aim of the study was to comparatively analyze the in vitro cell adhesion between nano coated titanium dioxide, and calcium hydroxyapatite (HA) coated titanium samples.
Materials and Methods:
Nano coated titanium dioxide, and calcium HA were coated onto the titanium samples by drop casting with NAFION membrane and cell culture was done by seeding human osteoblastic sarcoma cells on the coated samples.
Results and conclusion:
There was marked cell adhesion seen in the samples coated by titanium dioxide nano particles and more cells spreading as compared to calcium HA nano particles.
Keywords: Calcium hydroxyapatite, cyclic voltammetry, elemental analysis, fluorescence microscopy, human osteoblastic sarcoma cells, NAFION membrane, nano coated titanium dioxide
INTRODUCTION
The subversionary thoughts of many researchers in the field of implants have resulted in an unprecedented rise of several implant surfaces and research. The osteoblastic cell behavior acts as a yardstick to assess the success of such surfaces. The quest for better osseointegration has not only fuelled the demand of more implant surfaces, but also has evoked interest in the field of nanotechnology.
The extensive work by the Swedish orthopedic surgeon P.I. Brånemark led to the discovery that commercially pure titanium, when placed in a suitably prepared site in the bone, could become fixed in place due to a close bond that developed between the two, a phenomenon that he later described as osseointegration. This state has anatomical and functional dimensions. The present studies have shown that the components of the initial healing cascade are on the nanometer scale, and the binding sites where they act are even smaller. The lamellar bone formed at the interface after an adequate healing phase exhibits nanostructures such as abundant collagen fibrils and apatite crystals.[1,2]
There were many studies undertaken for improving the implant surface, but still more studies are needed to specify the ideal implant surface for better osseointegration and faster healing. Surface modifications are done on the surface substrate on endless applications, primarily to improve surface properties of the substrate, including binding, adhesion, corrosion, wear resistance, and scratch resistance. A “nano” coating is a controlled process at the nano level to significantly enhance the ability of a coating to improve surface properties. Nano coatings can be applied with chemical vapor deposition, pulse laser deposition, electron beam deposition, and electroplating each with its own advantages and disadvantages. Ultimately, the success and quality of nano coatings, on a given substrate, must be reliable and controlled.
It was hypothesized when the nano coatings stimulate the chemical composition of natural bone; the nanometer surface features will enhance bone formation compared to materials with conventional (or micron-scale) surface features. The recent studies have showcased the importance of nano network titanium dioxide coating on titanium surfaces to improve the osseointegration rate.[3] Hence, the aim of the study was to comparatively analyze the in vitro cell adhesion between nano coated titanium dioxide, and calcium hydroxyapatite (HA) coated titanium samples.
MATERIALS AND METHODS
In this study, the Grade II titanium samples were sectioned into 60 discs and grouped into three groups of 20 samples. The nano particles of titanium dioxide and calcium HA were dispersed in 2% NAFION anion exchange membrane and coated on to the surfaces of titanium discs. The coated samples were then cultured with human osteoblastic sarcoma (HOS) cells for 48 h and fixed with 2.5% glutaraldehyde. The scanning electron microscopy (SEM) analysis with fluoroscope microscopy was done to evaluate the cell adhesion.
The three groups of samples were the following:
Group A - Untreated machined
Group B - Titanium dioxide nano oxide coated
Group C - Calcium HA nano coated.
The uncoated machined samples were assigned as a control group [Figure 1].
Figure 1.

Simple machined samples
Coating of nano particles of titanium dioxide and hydroxyapatite
Coating was done by drop-casting method. It is the simplest method used to create thin films from solution using centrifugal force. Small amount of polymer solution (or chemical solution) was dropped onto spinning head (mold or surface) and depending on the evaporation kinetics and particle interaction with liquid-air interface, the morphology of the nano particle film can be controlled. The nano particle islands nucleate and grow as a result of rapid early-stage evaporation producing a two-dimensional solution of nano particles at the liquid-air interface, provided there is a favorable particle-interface interaction.[4]
Two milliliter of 1% NAFION anion-exchange membrane was dispersed along with 200 mg of TiO2 nano particles for a period of 30 min. The resultant mixture was drop casted on to the titanium disk of 6 mm diameter and 2 mm in thickness and kept for drying overnight. The nano scale cavities present in the NAFION membrane is able to hold the nano particles tightly.
NAFION membrane consists of an equal distribution of sulfonate ion clusters of 40 Å (4 nm) diameter held within a continuous fluorocarbon lattice and the clusters are interconnected by narrow channels of diameter 10 Å (1 nm) [Figure 2].
Figure 2.
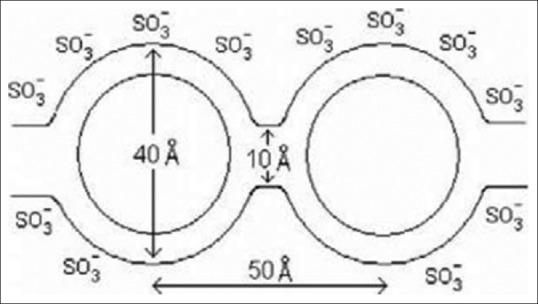
The architecture of NAFION membrane
Titanium dioxide nano particle coated samples
The titanium discs were coated with spherical titanium dioxide nano particles by drop-casting method. Anatase type of titanium dioxide nano particles was used for the study. Discs were treated with titanium dioxide nano particles. The formation of titanium dioxide nano particles, as well as their morphological dimensions in the SEM study, demonstrated that the average size was from 100 to 150 nm with inter-particle distance, whereas the shapes were uniformed spherical [Figure 3].
Figure 3.

The coated nano titanium dioxide sample and the scanning electron microscopy of the nano titanium dioxide
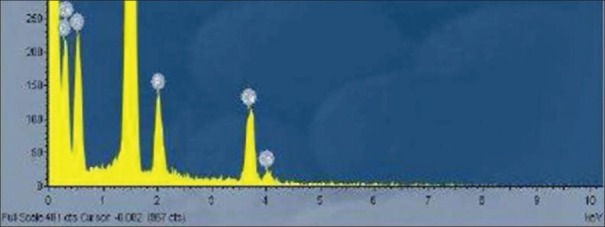
Energy-dispersive X-ray (EDX) spectroscopy (EDS or EDX or EDAX) is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on the investigation of the interaction of X-ray excitation and a sample. Its characterization capabilities are due in large part to the fundamental principle that when high energy electron or proton is focused on to an element, the movement of the electron from high energy outer shell to lower energy inner shell results in release of X-ray which is specific to a particular element and is recorded by an energy-dispersive spectrometer.
Elemental analysis of the coated titanium dioxide
Figure 4 shows even after 100 continuous cycles the nano TiO2 is held tight on the titanium disk with the help of the NAFION membrane. The nano scale cavities present the NAFION membrane is able to hold the nanoparticles tightly.
Figure 4.
Even after 100 continuous cycles the nano TiO2 is held tight on the titanium disk with the help of the NAFION membrane
Calcium hydroxyapatite nano particle coated samples.
The titanium discs were coated with calcium HA nano particles by drop-casting method. The SEM analysis showed that nano HA particles were having an appearance of needle-like crystal that characterizes HA crystals [Figures 5 and 6].
Figure 5.
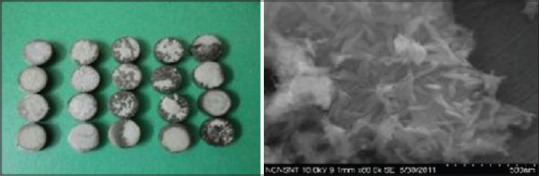
Nano calcium hydroxyapatite coated sample and scanning electron microscopy analysis of the sample
Figure 6.
Energy dispersive spectroscopy for nano calcium hydroxyapatite particles
Cell culture
Cell adhesion test was performed with test materials nanoparticles - (TiO2), Nano particles - (HaP) and control group of uncoated titanium disc. HOS cells were seeded on test materials at density of 1 × 104 cells/cm2 and incubated for 48 h at 37 + 1°C under humidified atmosphere containing 5% CO2. After 48 h cell-seeded the test materials and glass cover slips were fixed with 2.5% glutaraldehyde.
Scanning electron microscope: Analysis
The dry specimen is usually mounted on a specimen stub using an adhesive such as epoxy resin or electrically conductive double-sided adhesive tape, and sputter-coated with gold before examination in the microscope. The portion of the image that appears acceptably in focus is called the “depth of field.” The first field of interest was chosen by automatically setting the specimens to a central position in the microscope. The following consecutive micrograph was then taken in one direction. In this study, for each group, micrographs were taken, two micrographs before cell culture and two micrographs following cell culture. They were analyzed under magnification from × 500 to × 5000.
RESULTS
Control with osteoblastic cell growth.
The SEM analysis demonstrated polygonal osteoblastic cells with filopodial attachments and growth. As compared to nano coated samples of HA and titanium dioxide, it showed less colonies of osteoblastic cell growth. Development of radiate cell filopodia was not that apparent as in the case of nano coated HA [Figure 7].
Figure 7.
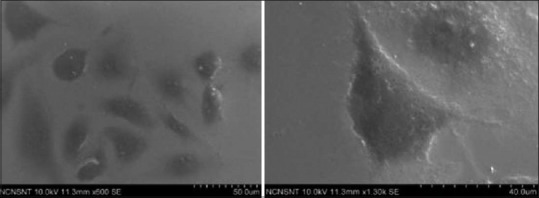
Scanning electron microscopy analysis showing osteoblastic cell adhesion for control
Calcium hydroxyapatite nano particles with osteoblastic cell growth
The SEM analysis showed that osteoblastic cell was deposited on the surfaces of nano coated HA. Development of radiate cell filopodia was more apparently seen. It showed more filopodial attachments than the control and nano coated titanium surfaces [Figure 8].
Figure 8.
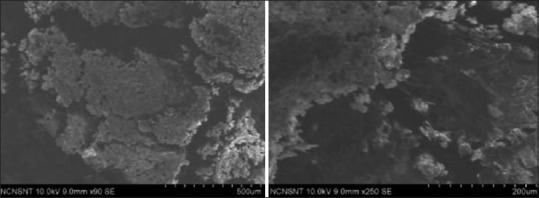
Scanning electron microscopy analysis shows osteoblastic cell adhesion for nano calcium hydroxyapatite
Titanium dioxide nano particle samples with osteoblastic cell growth
The SEM analysis showed more osteoblastic colonies with characteristic cell morphology.
Prominent looking osteoblastic cells were scattered throughout the sample and showed better adhesion as compared to control and nano coated HA group. Development of radiate cell filopodia was also seen. Osteoblast-like cells attach and spread well. These cells have been observed to grow into the pores of the metal surface and form an extracellular matrix [Figure 9].
Figure 9.
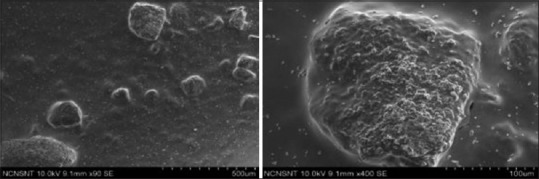
Scanning electron microscopy analysis shows osteoblastic cell adhesion for nano titanium dioxide
Fluorescence microscopy
The protein adsorption and fluorescence spectroscopy further confirmed that the titanium dioxide nano coated samples showed wider spread osteoblastic cell growth than the control and the calcium hydroxyapatite nano coated samples [Figure 10].
Figure 10.
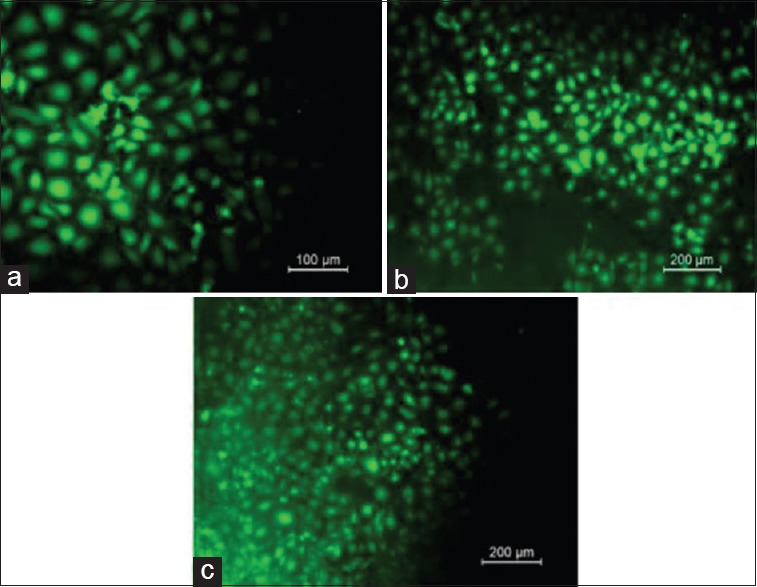
Fluorescence spectroscopy as seen in (a) control, (b) nano coated calcium hydroxide apatite and (c) nano coated titanium dioxide
There was cell adhesion seen on all the samples. SEM analysis showed titanium dioxide nano particle coated samples having marked osteoblastic cell colonies as compared to control and HA nano particles coated samples.
DISCUSSION
In the past studies have shown substratum composition and microtopography are important factors influencing growth and differentiation of osteoblasts. Most commercially available oral implants are moderately rough on the micrometer scale,[5] but no specific attention has been given to investigate an implant with nanostructures. The present study was designed so as to foresee enhanced osseointegration on nano textured implant. Clinical literature indicates increased osteoblastic cell growth on nano surfaces. Jäger et al. in 2007[6] found that Nanostructured surfaces enhance the surface area of biomaterials and promote cellular adherence. Zhao et al. in 2010[7] had compared nano topography with microtopography, and found the enhancement of multiple cell functions from the hierarchical micro/nano textured surfaces which lead to faster bone maturation around the titanium implants without compromising the bone mass. In addition, the hierarchical micro/nano textured surfaces retained the mechanical interlocking ability of the micro topography thereby bonding well for osseointegration Zhu et al.[8] stated that porous structures at either micro- or sub-micrometer scale supply positive guidance cues for anchorage-dependent cells to attach, leading to enhanced cell attachment in contrast, the cells attached to a smooth titanium surface by focal contacts around their periphery but as predominant adhesion structures, send repulsive signals from the environment and lead to retraction of the filopodia back to the cell bodies. These studies have indicated that nanotextured surfaces are better than micro surfaces. The regulation of bone formation and resorption (bone remodeling) is affected in old age as a result of the decrease in the number of osteoblastic cells.
Osteoblasts have demonstrated a biological dilemma where there exists an inverted correlation between proliferation and differentiation rates. Proliferation and differentiation are regulated by growth factors that counteract each other in osteoblasts. This applies to the bone formation around biomaterials. The bone mass, however, is smaller than that around the machined surface, due to the diminished osteoblastic proliferation. To compensate for the reduced rate of proliferation in the differentiating osteoblasts, the treatment of the cells with growth factors, e.g., transforming growth factors, increasing binding sites that specifically stimulate their proliferation, may be a biological strategy.[8,9]
To improve the osseointegration and bone response to titanium implants, an enhanced proliferation and differentiation of undifferentiated mesenchymal cells, osteoprogenitor cells, and preosteoblasts into osteoblasts is necessary.[11] The adhesion of plasma proteins on the surface of titanium implants has been reported to play an essential role in the process of osseointegration.[12] The specific pattern of adsorbed proteins determines the type of tissue that will develop at the interface between the implanted material and the host[13] cytokines have also been suggested to stimulate a deposition of cells with the capacity of regenerating the desired tissue (Liu et al., 2007, Sigurdsson et al., 1996).[14]
Nano calcium hydroxyapatite coated titanium surfaces
Hydroxyapatite is chemically similar to the mineral component of bones and teeth. It is among the few bioactive materials, meaning that it will support bone in growth and osseointegration when used in orthopedic, dental and maxillofacial applications. HA nano coatings are most commonly applied to metallic implants like stainless steel or titanium alloys.
In terms of cell adherence in this study, the nano HA have shown pronounced radiating filopodial attachments and better osteoblastic cell colonies than the control. The property of hydroxyl apatite favors the formation of direct and strong bond between the implant and bone tissue. The HA coating (Ca10(PO4) 6(OH) 2) is considered as bioactive because of the sequence of events that results in precipitation of a CaP rich layer on the implant material through a solid solution ion exchange at the implant bone interface.[15] The CaP incorporated layer will gradually be developed, via octacalcium phosphate, in a biologically equivalent HA that will be incorporated in the developing bone.[16] Ducheyne and Cuckler in 1992 studied the synthetic form of HA due its similar composition to the mineral matrix of the bone. Hee Lee and Kim[17] mentioned in his studies that fragmentation and breakdown of the HA coated layer and its mechanical instability leads to an acute inflammatory reaction. Barrere et al. stated that the surface roughness did not affect the development of the HA globules. However, a rougher substrate allowed the final HA coating to remain on the surface, whereas a smoother substrate was not efficient for the anchorage of the final HA coating.
Nano titanium dioxide coated titanium samples
In this study, prominent looking osteoblastic cells were scattered throughout the sample and showed better adhesion as compared to control and nano coated HA group. Development of radiate cell filopodia was also seen. The topography and surface oxide layer of the implant can be altered by means of an electrochemical process called anodic oxidation. It increases the TiO2 surface layer and roughness thereby changing the characteristic of the oxide layer and makes it more biocompatible.[18] The process is similar to an electrochemical cell where, implant is placed in a suitable electrolytic solution and acts as the anode. When a potential is applied, there is deposition of oxide film on the anode (implant) as a result of ionic transport of charge.[19] This results in a surface with micropores which demonstrates increased cell attachment and proliferation.
Chiang et al.[19] has demonstrated the formation of multilayer TiO2 nano network on the titanium surface using a simple electrochemical anodization treatment. This network layer significantly improved in vitro and in vivo human mesenchymal stem cells (HMSC) growth, as assessed by measurement of green fluorescent protein fluorescence, relative to HMSC growth on untreated Ti surface. For successful cell growth, TiO2 nanotubes with lateral openings 30–50 nm in diameter are critical, as it favors integrin clustering and the formation of focal adhesion complexes.
CONCLUSION
The cell adhesion seen in simple machined samples were having less spreading of the human osteoblastic cells and had fewer osteoblastic cell colonies
The cell adhesion seen in nano calcium HA coated sample was having more and distinct spreading of cells. Development of radiate cell filopodia was more apparently seen. It showed more filopodial attachments than the control and nano coated titanium surfaces. However, they had fewer osteoblastic cell colonies
The cell adhesion seen in nano titanium dioxide coated samples were showing more prominent looking osteoblastic cells were scattered throughout the sample and showed better adhesion as compared to control and nano coated HA group. Development of radiate cell filopodia was also seen.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Adams JC. Cell-matrix contact structures. Cell Mol Life Sci. 2001;58:371–92. doi: 10.1007/PL00000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrektsson T, Wennerberg A. Oral implant surfaces: Part 2 - Review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004;17:544–64. [PubMed] [Google Scholar]
- 3.Aumailley M, Gayraud B. Structure and biological activity of the extracellular matrix. J Mol Med (Berl) 1998;76:253–65. doi: 10.1007/s001090050215. [DOI] [PubMed] [Google Scholar]
- 4.Bigioni TP, Lin XM, Nguyen TT, Corwin EI, Witten TA, Jaeger HM. Kinetically driven self assembly of highly ordered nanoparticle monolayers. Nat Mater. 2006;5:265–70. doi: 10.1038/nmat1611. [DOI] [PubMed] [Google Scholar]
- 5.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–22. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 6.Jäger M, Zilkens C, Zanger K, Krauspe R. Significance of nano-and microtopography for cell-surface interactions in orthopaedic implants. J Biomed Biotechnol 2007. 2007 doi: 10.1155/2007/69036. 69036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L, Mei S, Chu PK, Zhang Y, Wu Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials. 2010;31:5072–82. doi: 10.1016/j.biomaterials.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Chen J, Scheideler L, Altebaeumer T, Geis-Gerstorfer J, Kern D. Cellular reactions of osteoblasts to micron-and submicron-scale porous structures of titanium surfaces. Cells Tissues Organs. 2004;178:13–22. doi: 10.1159/000081089. [DOI] [PubMed] [Google Scholar]
- 9.Barrere F, Snel MM, van Blitterswijk CA, de Groot K, Layrolle P. Nano-scale study of the nucleation and growth of calcium phosphate coating on titanium implants. Biomaterials. 2004;25:2901–10. doi: 10.1016/j.biomaterials.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 10.Chappard D, Aguado E, Huré G, Grizon F, Basle MF. The early remodeling phases around titanium implants: A histomorphometric assessment of bone quality in a 3-and 6-month study in sheep. Int J Oral Maxillofac Implants. 1999;14:189–96. [PubMed] [Google Scholar]
- 11.Eriksson C, Lausmaa J, Nygren H. Interactions between human whole blood and modified TiO2-surfaces: Influence of surface topography and oxide thickness on leukocyte adhesion and activation. Biomaterials. 2001;22:1987–96. doi: 10.1016/s0142-9612(00)00382-3. [DOI] [PubMed] [Google Scholar]
- 12.Wälivaara B, Aronsson BO, Rodahl M, Lausmaa J, Tengvall P. Titanium with different oxides: In vitro studies of protein adsorption and contact activation. Biomaterials. 1994;15:827–34. doi: 10.1016/0142-9612(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 13.Sigurdsson TJ, Nygaard L, Tatakis DN, Fu E, Turek TJ, Jin L, et al. Periodontal repair in dogs: Evaluation of rhBMP-2 carriers. Int J Periodontics Restorative Dent. 1996;16:524–37. [PubMed] [Google Scholar]
- 14.Ducheyne P, Cuckler JM. Bioactive ceramic prosthetic coatings. Clin Orthop Relat Res. 1992;276:102–14. [PubMed] [Google Scholar]
- 15.Ogiso M, Tabata T, Ichijo T, Borgese D. Examination of human bone surrounded by a dense hydroxyapatite dental implant after long-term use. J Long Term Eff Med Implants. 1992;2:235–47. [PubMed] [Google Scholar]
- 16.Hee Lee S, Kim HE. Nano-sized hydroxyapatite coatings on Ti substrate with TiO2 buffer layer by E-beam deposition. Journal of the American Ceramic Society. 2007;90:50–6. [Google Scholar]
- 17.Gupta A, Dhanraj M, Sivagami G. Status of surface treatment in endosseous implant: A literary overview. Indian J Dent Res. 2010;21:433–8. doi: 10.4103/0970-9290.70805. [DOI] [PubMed] [Google Scholar]
- 18.Anil S, Anand P S, Alghamdi H, Jansen J A. Prof. Ilser Turkyilmaz., editor. Dental Implant Surface Enhancement and Osseointegration, InTech: Implant Dentistry - A Rapidly Evolving Practice. ISBN: 978-953-307-658-4. DOI: 10.5772/16475. Available from: http://www.intechopen.com/books/implant-dentistry-a-rapidly-evolving-practice/dental-implant-surfaceenhancement-and-osseointegration .
- 19.Chiang CY, Chiou SH, Yang WE, Hsu ML, Yung MC, Tsai ML, et al. Formation of TiO (2) nano-network on titanium surface increases the human cell growth. Dent Mater. 2009;25:1022–9. doi: 10.1016/j.dental.2009.03.001. [DOI] [PubMed] [Google Scholar]