Abstract
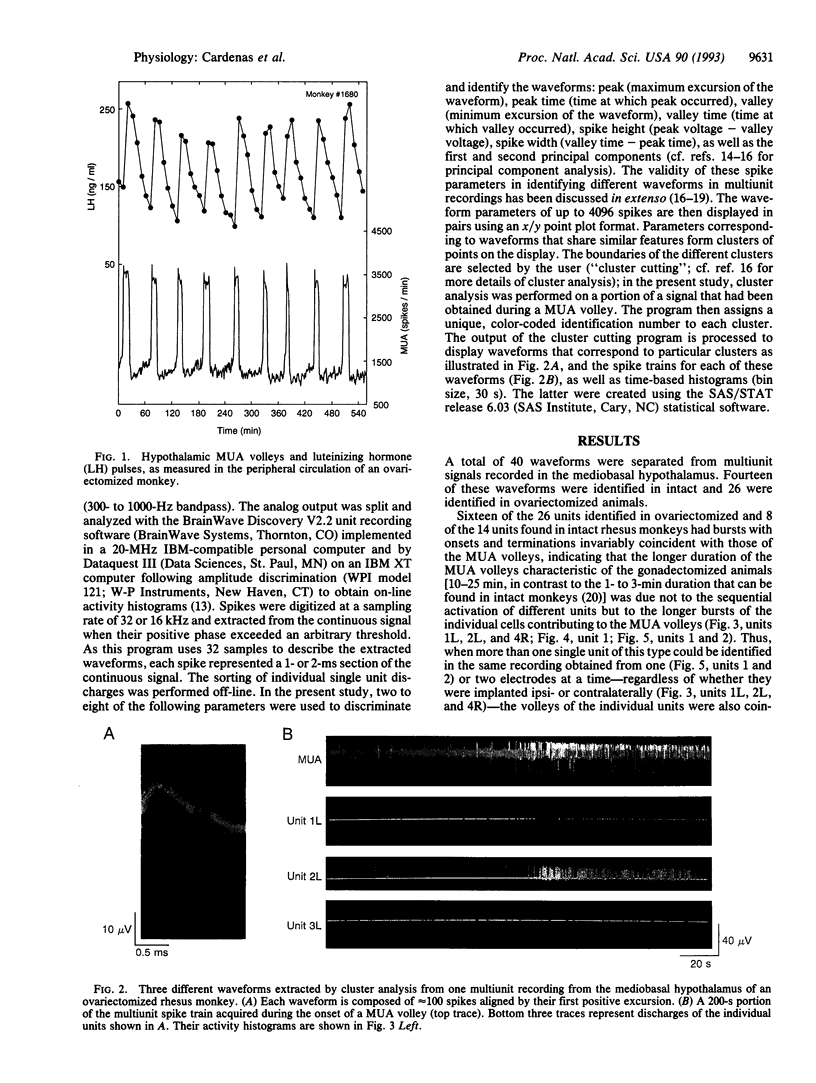
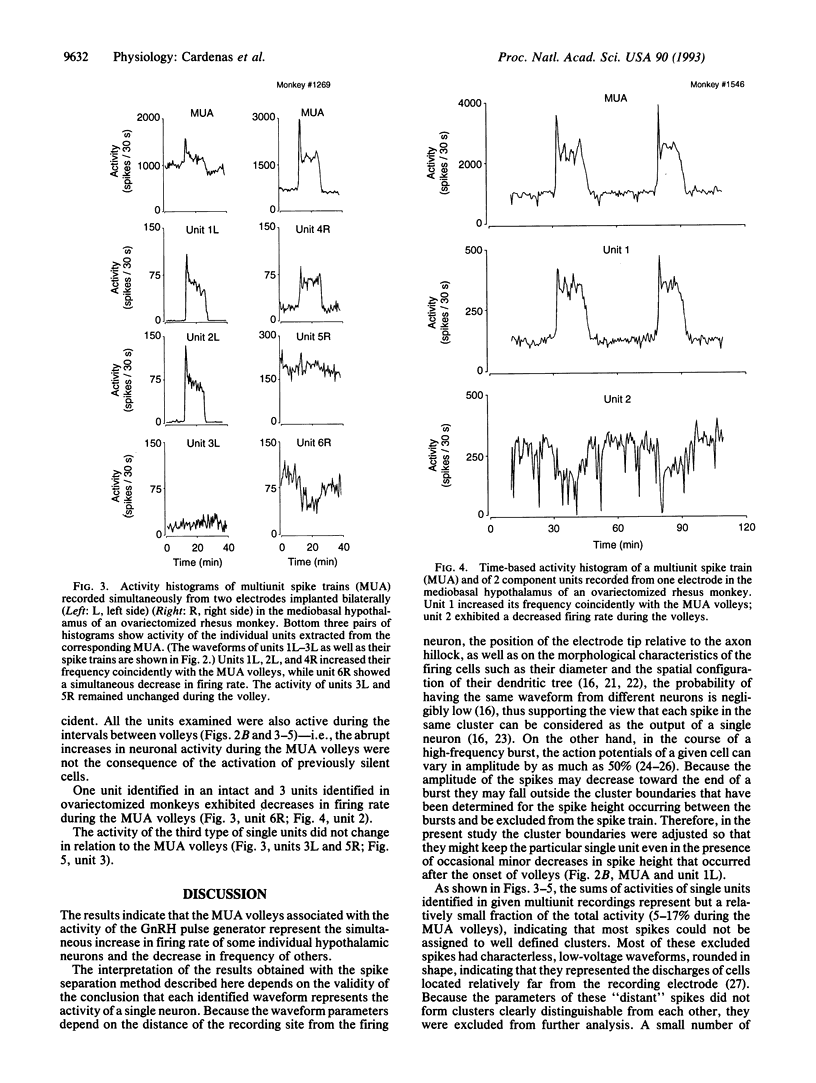
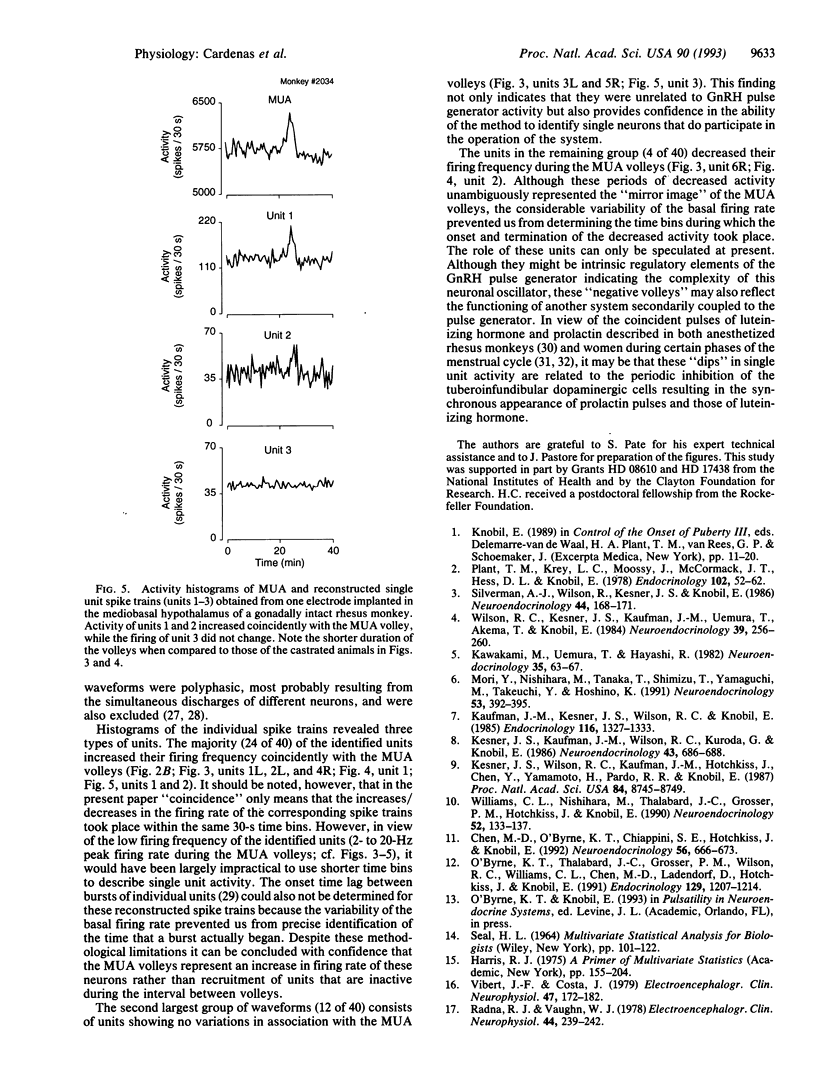
Vertebrate reproduction is dependent on the operation of a central signal generator that directs the episodic release of gonadotropin-releasing hormone, a neuropeptide that stimulates secretion of the pituitary gonadotropic hormones and, thereby, controls gonadal function. The electrophysiological correlates of this pulse generator are characterized by abrupt increases in hypothalamic multiunit electrical activity (MUA volleys) invariably associated with the initiation of secretory episodes of luteinizing hormone. Using cluster analysis, we extracted single units from the multiunit signals recorded from the mediobasal hypothalamus of four intact and four ovariectomized rhesus monkeys. Of the 40 individual units identified in this manner, 24 increased their frequency with the MUA volleys. The onset and termination of these single-unit bursts occurred coincidently with those of the MUA volleys in both intact and ovariectomized animals, indicating that the longer duration of the MUA volleys characteristic of the gonadectomized animals was due not to the sequential activation of different units but to the longer bursts of the individual cells. Four other units showed decreases in firing rate during the MUA volleys, while the frequency of the remainder did not change. All the examined units were active during the intervals between the volleys of electrical activity. The results indicate that the MUA volleys associated with the activity of the gonadotropin-releasing hormone pulse generator represent the simultaneous increase in firing rate of some individual hypothalamic neurons and the decrease in the frequency of others.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belchetz P., Dufy B., Knobil E. Identification of inhibitory and stimulatory control of prolactin secretion in the rhesus monkey. Neuroendocrinology. 1978;27(1-2):32–38. doi: 10.1159/000122798. [DOI] [PubMed] [Google Scholar]
- Belin V., Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986 Aug;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper R. F., Yen S. S. Simultaneous pulsatile release of prolactin and luteinizing hormone induced by luteinizing hormone-releasing factor agonist. J Clin Endocrinol Metab. 1981 May;52(5):934–936. doi: 10.1210/jcem-52-5-934. [DOI] [PubMed] [Google Scholar]
- Chen M. D., O'Byrne K. T., Chiappini S. E., Hotchkiss J., Knobil E. Hypoglycemic 'stress' and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology. 1992 Nov;56(5):666–673. doi: 10.1159/000126291. [DOI] [PubMed] [Google Scholar]
- Clifton D. K., Aksel S., Bremner W. J., Steiner R. A., Soules M. R. Statistical evaluation of coincident prolactin and luteinizing hormone pulses during the normal menstrual cycle. J Clin Endocrinol Metab. 1988 Oct;67(4):832–838. doi: 10.1210/jcem-67-4-832. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961 May;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- Kaufman J. M., Kesner J. S., Wilson R. C., Knobil E. Electrophysiological manifestation of luteinizing hormone-releasing hormone pulse generator activity in the rhesus monkey: influence of alpha-adrenergic and dopaminergic blocking agents. Endocrinology. 1985 Apr;116(4):1327–1333. doi: 10.1210/endo-116-4-1327. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Uemura T., Hayashi R. Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology. 1982;35(1):63–67. doi: 10.1159/000123356. [DOI] [PubMed] [Google Scholar]
- Kesner J. S., Kaufman J. M., Wilson R. C., Kuroda G., Knobil E. The effect of morphine on the electrophysiological activity of the hypothalamic luteinizing hormone-releasing hormone pulse generator in the rhesus monkey. Neuroendocrinology. 1986;43(6):686–688. doi: 10.1159/000124605. [DOI] [PubMed] [Google Scholar]
- Kesner J. S., Wilson R. C., Kaufman J. M., Hotchkiss J., Chen Y., Yamamoto H., Pardo R. R., Knobil E. Unexpected responses of the hypothalamic gonadotropin-releasing hormone "pulse generator" to physiological estradiol inputs in the absence of the ovary. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8745–8749. doi: 10.1073/pnas.84.23.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter A. K., Aertsen A. M., Gerstein G. L. A low-cost single-board solution for real-time, unsupervised waveform classification of multineuron recordings. J Neurosci Methods. 1989 Oct;30(1):59–69. doi: 10.1016/0165-0270(89)90075-7. [DOI] [PubMed] [Google Scholar]
- Meister M., Wong R. O., Baylor D. A., Shatz C. J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991 May 17;252(5008):939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Mori Y., Nishihara M., Tanaka T., Shimizu T., Yamaguchi M., Takeuchi Y., Hoshino K. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology. 1991 Apr;53(4):392–395. doi: 10.1159/000125746. [DOI] [PubMed] [Google Scholar]
- O'Byrne K. T., Chen M. D., Nishihara M., Williams C. L., Thalabard J. C., Hotchkiss J., Knobil E. Ovarian control of gonadotropin hormone-releasing hormone pulse generator activity in the rhesus monkey: duration of the associated hypothalamic signal. Neuroendocrinology. 1993 Apr;57(4):588–592. doi: 10.1159/000126411. [DOI] [PubMed] [Google Scholar]
- O'Byrne K. T., Thalabard J. C., Grosser P. M., Wilson R. C., Williams C. L., Chen M. D., Ladendorf D., Hotchkiss J., Knobil E. Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology. 1991 Sep;129(3):1207–1214. doi: 10.1210/endo-129-3-1207. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. M., Krey L. C., Moossy J., McCormack J. T., Hess D. L., Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology. 1978 Jan;102(1):52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- RALL W. Electrophysiology of a dendritic neuron model. Biophys J. 1962 Mar;2(2 Pt 2):145–167. doi: 10.1016/s0006-3495(62)86953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radna R. J., Vaughn W. J. Computer assisted unit data acquistion/reduction. Electroencephalogr Clin Neurophysiol. 1978 Feb;44(2):239–242. doi: 10.1016/0013-4694(78)90271-7. [DOI] [PubMed] [Google Scholar]
- Ranck J. B., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973 Nov;41(2):461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Rosenthal F., Woodbury J. W., Patton H. D. Dipole characteristics of pyramidal cell activity in cat postcruciate cortex. J Neurophysiol. 1966 Jul;29(4):612–625. doi: 10.1152/jn.1966.29.4.612. [DOI] [PubMed] [Google Scholar]
- Schmidt E. M. Computer separation of multi-unit neuroelectric data: a review. J Neurosci Methods. 1984 Dec;12(2):95–111. doi: 10.1016/0165-0270(84)90009-8. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Wilson R., Kesner J. S., Knobil E. Hypothalamic localization of multiunit electrical activity associated with pulsatile LH release in the rhesus monkey. Neuroendocrinology. 1986;44(2):168–171. doi: 10.1159/000124641. [DOI] [PubMed] [Google Scholar]
- Vibert J. F., Albert J. N., Costa J. Intelligent software for spike separation in multiunit recordings. Med Biol Eng Comput. 1987 Jul;25(4):366–372. doi: 10.1007/BF02443355. [DOI] [PubMed] [Google Scholar]
- Vibert J. F., Costa J. Spike separation in multiunit records: a multivariate analysis of spike descriptive parameters. Electroencephalogr Clin Neurophysiol. 1979 Aug;47(2):172–182. doi: 10.1016/0013-4694(79)90219-0. [DOI] [PubMed] [Google Scholar]
- Williams C. L., Nishihara M., Thalabard J. C., Grosser P. M., Hotchkiss J., Knobil E. Corticotropin-releasing factor and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Electrophysiological studies. Neuroendocrinology. 1990 Aug;52(2):133–137. doi: 10.1159/000125563. [DOI] [PubMed] [Google Scholar]
- Wilson R. C., Kesner J. S., Kaufman J. M., Uemura T., Akema T., Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984 Sep;39(3):256–260. doi: 10.1159/000123988. [DOI] [PubMed] [Google Scholar]



