Abstract
Background
Blood pressure (BP) control may have different effects on cardiovascular (CV) and renal outcomes in diabetes. We examined the impact of systolic BP (SBP) on renal and CV outcomes in a post hoc analysis in the Olmesartan Reducing Incidence of Endstage Renal Disease in Diabetic Nephropathy Trial.
Methods
We stratified mean follow-up SBP into three categories (≤130, 131–140 and >140 mmHg) and used a Cox regression model to estimate the hazard ratio (HR, 95% confidence interval) for the outcomes. The composite renal outcome was doubling of serum creatinine, end-stage renal disease and all-cause death. The composite CV outcome included CV death, nonfatal stroke, nonfatal myocardial infarction, hospitalization for unstable angina or heart failure, revascularization and lower extremity amputation. We also compared the slope of estimated glomerular filtration rate (eGFR) in all three groups.
Results
After a mean follow-up period of 3.2 years, the follow-up SBP was linearly associated with risk of renal outcomes in all 566 patients. In patients with heavy proteinuria (≥1 g/gCr), a follow-up SBP > 130 mmHg was associated with an HR of 2.33 (1.62–3.36) for renal outcomes with referent to SBP ≤ 130 mmHg. In patients without history of CV disease, a follow-up SBP > 140 mmHg was associated with an HR of 2.04 (1.23–3.40) for CV outcomes with referent to SBP < 140 mmHg. The median (interquartile range) slopes of eGFR were −3.27 (−6.90, −1.63), −4.53 (−8.08, −2.29) and −7.13 (−10.90, −3.99) dL/mg/year in patients with SBP ≤ 130, 131–140 and > 140 mmHg, respectively (P = 0.008 between ≤130 and 131–140, P < 0.001 between ≤ 130 and > 140 mmHg).
Conclusion
In Asian type 2 diabetic patients with chronic kidney disease and heavy proteinuria, reduction of SBP ≤ 130 mmHg was associated with greater renoprotection than cardioprotection. However, our results emphasize the need to individualize BP targets in type 2 diabetes.
Keywords: cardiovascular outcome, diabetic nephropathy, proteinuria, renal outcome, systolic blood pressure
INTRODUCTION
The target blood pressure (BP) for patients with type 2 diabetes and chronic kidney disease (CKD) remains controversial [1–3]. In epidemiological surveys, high BP was associated with increased rates of premature mortality, cardiovascular (CV) diseases and progressive loss of kidney function in patients with hypertension and/or diabetes [4–6]. To date, only two randomized controlled trials have examined the effects of BP lowering on CV events (stroke and coronary heart disease) and related deaths in type 2 diabetes. In the United Kingdom Prospective Diabetes Study (UKPDS 38), targeting systolic BP (SBP) < 150 mmHg (achieved SBP of 144 mmHg) reduced CV outcome and progression of nephropathy in Caucasian patients with type 2 diabetes and hypertension [7]. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study, intensive treatment targeting SBP < 120 mmHg failed to reduce CV outcome, except for stroke, compared with standard therapy targeting SBP < 140 mmHg. Although intensive BP control reduced albuminuria, there was a significant increase in serum creatinine (SCr) especially in patients with estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 [8]. Results from these landmark studies have led to the recommendation of a target BP of 140/80 mmHg in type 2 diabetes by the American Diabetes Association [9]. On the other hand, the Kidney Disease Improving Global Outcomes (KDIGO) Guidelines continued to recommend a BP ≤ 130/80 mmHg in diabetic patients with hypertension with microalbuminuria but ≤140/90 mmHg in those with normoalbuminuria [10], while the Joint National Committee (JNC) 8 Hypertension Guidelines recommended lowering SBP to <140 mmHg in patients younger than 60 [11]. Apart from the controversial effects of SBP on CV and renal outcomes [12, 13], most of these recommendations were based on data from white and black populations and might not be applicable to Asian patients with diabetes.
In a meta-analysis of observational cohorts of 1 million people, lower BP was associated with lower risk of stroke with no apparent threshold [4]. Compared with their Caucasian counterparts, Asian patients were more prone to develop stroke and renal disease and less likely to develop coronary heart disease although the latter was strongly predicted by renal impairment [14]. Given the controversies on current guidelines on BP target as well as interethnic differences in propensity for different clinical outcomes, we conducted a post hoc analysis of the ORIENT (Olmesartan Reducing Incidence of Endstage renal disease in diabetic Nephropathy Trial) to explore the optimal SBP level for prevention of renal and CV outcome in Asian type 2 diabetic patients with renal impairment and heavy proteinuria [15].
MATERIALS AND METHODS
Patients and study design
We screened 857 patients and enrolled 377 patients from Japan and 200 patients from Hong Kong with the following inclusion criteria: (i) age between 30 and 70 years, (ii) urinary albumin/creatinine ratio (UACR) > 300 mg/gCr in the first morning urine sample and (iii) SCr of 1.0–2.5 mg/dL in female and 1.2–2.5 mg/dL in male. The major exclusion criteria included (i) type 1 diabetes, (ii) history of myocardial infarction or coronary artery bypass grafting within 3 months prior to consent, (iii) percutaneous coronary intervention, carotid artery or peripheral artery revascularization within 6 months, (iv) stroke or transient ischemic attack within 1 year, (v) unstable angina pectoris or heart failure of New York Heart Association functional class III or IV, (vi) rapidly progressive renal disease within 3 months prior to consent, (vii) severe orthostatic hypotension and (viii) serum potassium level ≤3.5 or ≥5.5 mEq/L. This study was conducted with adherence to Good Clinical Practice and the Declaration of Helsinki. All patients provided signed informed consent. The protocol was approved by all participating institutions. The trial commenced in May 2003 and terminated in February 2008. SCr was measured at a central laboratory in Japan (SRL, Inc., Tokyo, Japan).
Measurement
In this post hoc analysis, we focused on the risk association of follow-up SBP with composite renal outcome. Sitting BP was measured twice in the clinic and the average value was used for analysis. Urinary protein/creatinine ratio (UPCR) was measured in the first morning urine sample collected at baseline. None of the enrolled patients had missing value of SBP and UPCR at baseline.
Statistical analysis
The prespecified primary analysis had been described [15]. Consistent with the prespecified analysis, post hoc analysis included all 566 randomized and eligible participants in the ORIENT. In this post hoc analysis, we evaluated association of renal or CV outcomes with follow-up SBP, which was the average SBP values from baseline to occurrence of the outcomes.
We examined the continuous relationships between SBP and occurrence of renal or CV outcomes by using the moving average method [16] that plotted the percentages of outcomes by every 2 mmHg within a SBP range of 120–160 mmHg. We plotted the percentages of composite renal outcome for mean follow-up SBP. Each plotted percentage of outcomes was calculated for patients whose SBP fell within the interval of −6 and +6 mmHg for a specific SBP. We also stratified patients using UPCR greater than or less than 1 g/gCr at baseline.
We stratified the mean follow-up SBP into three categories (≤130, 131–140 and >140 mmHg) and used Cox regression model to estimate the hazard ratio (HR) of renal and CV outcomes with 95% confidence interval (CI) using ≤130 mmHg as the referent group. The covariables in the Cox regression model included region (Japan or Hong Kong), SCr, age, gender, baseline UPCR, SBP, HbA1c, blood hemoglobin, total cholesterol and previous history of CV disease.
We calculated the HR for renal and CV outcomes with dichotomized categories (SBP into two categories: ≤130, >130 mmHg for renal and ≤140, >140 mmHg for CV outcomes), with the lower BP groups as the referent group. We also stratified patients using UPCR greater than or less than 1 g/gCr at baseline, and with or without previous history of CV disease, and then tested interactions of those strata and the dichotomized BP categories on each outcome with a significance level of 0.20. The Kaplan–Meier method was used to estimate the cumulative event rates by the two-level SBP categories and with or without previous history of CV disease [17]. The rate of reduction in eGFR was calculated by regression analysis of eGFR over time in each patient. We calculated eGFR by the Japanese equation and the Modification of Diet in Renal Disease (MDRD) equation with SCr in Japanese and Chinese patients, respectively [18, 19].
All values were expressed as mean ± SD or median (interquartile range) as appropriate. All statistical tests except for the interactions were two sided with 0.05 as significance level. Statistical analyses were performed using Statistical Analysis System version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study population
At enrollment, the mean age of the participants was 59.2 years (69.1% male). The mean SBP and diastolic BP (DBP) at baseline were 141.3 and 77.5 mmHg, respectively, and SCr, 1.62 mg/dL. The time-averaged SBP and DBP during follow-up were 137.6 and 75.0 mmHg, respectively. The median of baseline UPCR and UACR was 2.12 and 1.69 g/gCr, respectively. In this analysis which focused on risk association with SBP, the clinical profiles were similar among the three groups stratified by follow-up SBP (≤130, 131–140 and >140 mmHg) (Table 1). After a mean follow-up period of 3.2 years, 245 and 93 patients developed the composite renal and CV outcomes, respectively.
Table 1.
Baseline clinical and biochemical characteristics of Asian Type 2 diabetic patients with renal impairment and overt proteinuria, stratified by mean follow-up SBP in the ORIENT study
| Characteristics | All (n = 566) | Mean SBP (mmHg) during follow-up |
||
|---|---|---|---|---|
| ≤130 (n = 158) | 131–140 (n = 177) | >140 (n = 231) | ||
| Male, n (%) | 391 (69.1) | 111 (70.3) | 126 (71.2) | 154 (66.7) |
| Age (years) | 59.2 ± 8.1 | 58.4 ± 7.8 | 59.7 ± 8.3 | 59.2 ± 8.1 |
| Smoker, n (%) | 144 (25.4) | 35 (22.2) | 50 (28.2) | 59 (25.5) |
| Body weight (kg) | 66.4 ± 12.8 | 66.1 ± 12.2 | 66.8 ± 13.2 | 66.4 ± 12.9 |
| Body mass index (kg/m2) | 25.33 ± 4.03 | 25.10 ± 4.24 | 25.39 ± 3.84 | 25.45 ± 4.02 |
| SBP (mmHg) | 141.3 ± 17.5 | 129.2 ± 13.3 | 139.6 ± 14.4 | 150.7 ± 16.8 |
| DBP (mmHg) | 77.5 ± 10.5 | 73.9 ± 9.4 | 76.9 ± 10.5 | 80.3 ± 10.5 |
| UACR (g/gCr) | 1.69 (0.82–3.03) | 1.10 (0.66–2.17) | 1.52 (0.81–2.77) | 2.15 (1.17–3.77) |
| UPCR (g/gCr) | 2.12 (1.03–3.82) | 1.46 (0.84–2.82) | 1.89 (0.99–3.60) | 2.67 (1.47–4.79) |
| Serum creatinine (mg/dL) | 1.62 ± 0.34 | 1.61 ± 0.36 | 1.58 ± 0.29 | 1.66 ± 0.36 |
| Serum potassium (mEq/L) | 4.61 ± 0.42 | 4.65 ± 0.39 | 4.59 ± 0.42 | 4.59 ± 0.43 |
| Hemoglobin A1c (%) | 7.08 ± 1.22 | 7.06 ± 1.33 | 7.15 ± 1.24 | 7.04 ± 1.13 |
| Total cholesterol (mg/dL) | 208.3 ± 49.6 | 202.0 ± 44.8 | 202.7 ± 45.1 | 216.9 ± 54.7 |
| Blood hemoglobin (g/L) | 12.28 ± 1.95 | 12.30 ± 1.95 | 12.37 ± 2.03 | 12.19 ± 1.89 |
| Uric acid (mg/dL) | 7.25 ± 1.56 | 7.30 ± 1.51 | 7.22 ± 1.49 | 7.24 ± 1.64 |
| Medical history—n (%) | ||||
| Diabetic retinopathy | 461 (81.4) | 121 (76.6) | 143 (80.8) | 197 (85.3) |
| Diabetic neuropathy | 298 (52.7) | 82 (51.9) | 103 (58.2) | 113 (48.9) |
| Prior history of CV disease | 93 (16.4) | 27 (17.1) | 35 (19.8) | 31 (13.4) |
| Myocardial infarction | 16 (2.8) | 5 (3.2) | 8 (4.5) | 3 (1.3) |
| Coronary revascularization | 32 (5.7) | 7 (4.4) | 16 (9.0) | 9 (3.9) |
| Heart failure | 21 (3.7) | 4 (2.5) | 7 (4.0) | 10 (4.3) |
| Peripheral artery disease | 52 (9.2) | 16 (10.1) | 21 (11.9) | 15 (6.5) |
| Stroke or transient ischemic attack | 83 (14.7) | 18 (11.4) | 30 (16.9) | 35 (15.2) |
| Severe orthostatic hypotension | 8 (1.4) | 4 (2.5) | 2 (1.1) | 2 (0.9) |
| Antihypertensive treatment at baseline, n (%) | 531 (93.8) | 145 (91.8) | 168 (94.9) | 218 (94.4) |
| Angiotensin-converting enzyme inhibitors, n (%) | 414 (73.1) | 116 (73.4) | 126 (71.2) | 172 (74.5) |
| Calcium channel blockers, n (%) | 384 (67.8) | 87 (55.1) | 122 (68.9) | 175 (75.8) |
| Diuretics, n (%) | 207 (36.6) | 52 (32.9) | 62 (35.0) | 93 (40.3) |
| α-Blockers, n (%) | 82 (14.5) | 16 (10.1) | 23 (13.0) | 43 (18.6) |
| β-Blocker, n (%) | 96 (17.0) | 20 (12.7) | 35 (19.8) | 41 (17.7) |
| Others, n (%) | 75 (13.3) | 15 (9.5) | 28 (15.8) | 32 (13.9) |
Values are mean ± SD or median (interquartile range) or number (%).
Impacts of SBP on renal outcome
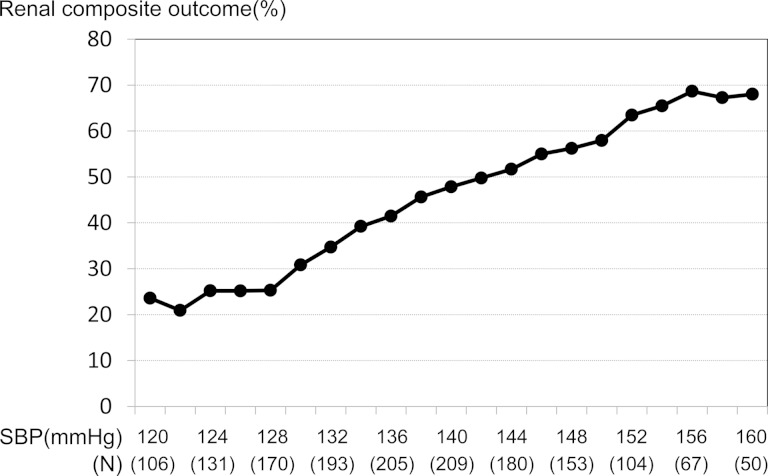
Increase in follow-up SBP was associated with increased incidence of renal outcome (Figure 1). The incidence of renal outcome declined with decreasing SBP, then leveled off at a value below 130 mmHg. Compared with patients with SBP ≤ 130 mmHg, the respective HR of renal outcome was 1.69 (1.14–2.51, P = 0.009) in patients with SBP 131–140 mmHg and 3.01 (2.11–4.30, P < 0.001) in those with SBP > 140 mmHg. The rate of decline of renal function as indicated by slope of eGFR was significantly slower in patients with SBP ≤ 130 and 131–140 mmHg compared with those with SBP > 140 mmHg. The beneficial effects were more prominent in olmesartan-treated patients with SBP ≤ 130 mmHg (Table 2).
FIGURE 1:
Association between renal composite outcome and follow-up SBP. The pattern plot shows the relationships between percentages of composite renal outcome and follow-up SBP. Each plotted percentage of outcomes is calculated for patients whose follow-up SBP lies within the interval between −6 and +6 mmHg for a specific SBP.
Table 2.
Yearly rate of change of eGFR stratified by mean follow-up SBP during a median follow-up period of 3.2 years
| SBP (mmHg) | All | Placebo | Olmesartan |
|---|---|---|---|
| ≤130 | −3.27 (−6.90, −1.63), n = 158 | −4.10 (−7.08, −2.33), n = 65 | −2.88*** (−6.65, −1.27), n = 93 |
| 131–140 | −4.53* (−8.08, −2.29), n = 172 | −4.89 (−8.07, −2.59), n = 89 | −4.03 (−8.72, −1.81), n = 83 |
| >140 | −7.13** (−10.90, −3.99), n = 236 | −7.00 (−10.68, −3.84), n = 130 | −7.34 (−11.49, −4.43), n = 106 |
Values are (dL/mg/year) [median (interquartile range)].
*P-value = 0.008 (SBP ≤ 130 versus 131–140 mmHg in all patients).
**P-value < 0.001 (SBP ≤ 130 versus > 140 mmHg in all patients).
***P-value = 0.016 (olmesartan versus placebo in SBP ≤ 130 mmHg).
Proteinuria, SBP and renal outcome
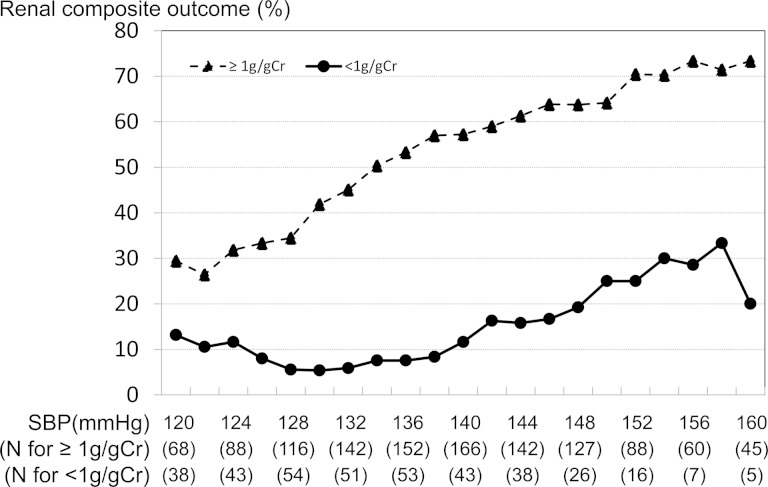
We stratified patients by baseline proteinuria at 1 g/gCr and studied the impact of SBP on incidence of renal outcome. Increases in follow-up SBP were associated with increased incidence of renal outcome in both groups of patients stratified by baseline proteinuria (Figure 2 and Table 3). In patients with baseline UPCR < 1 g/gCr, the HR for renal outcome was 1.25 (0.42–3.69, P = 0.687) in the >130 mmHg group compared with SBP ≤ 130 mmHg group. In patients with baseline UPCR ≥ 1 g/gCr, the corresponding HR was 2.33 (1.62–3.36, P < 0.001) with significant interaction (Table 3).
FIGURE 2:
Association between renal composite outcome and follow-up SBP stratified by baseline proteinuria. The pattern plot shows the relationships between percentages of composite renal outcome and follow-up SBP. Each plotted percentage of outcomes is calculated for patients whose follow-up SBP lies within the interval between −6 and +6 mmHg for a specific SBP.
Table 3.
Relationship between follow-up SBP and outcome and modification by baseline UPCR (renal composite outcome)
| n | Follow-up SBP (mmHg) |
P-value for interaction | ||
|---|---|---|---|---|
| ≤130 | >130 | |||
| UPCR | ||||
| <1 g/gCr | 136 | Reference | 1.25 (0.42–3.69) | 0.192* |
| ≥1 g/gCr | 430 | Reference | 2.33 (1.62–3.36) | |
Values are hazard ratio (95% CI).
*Significant interaction for a P-value <0.20.
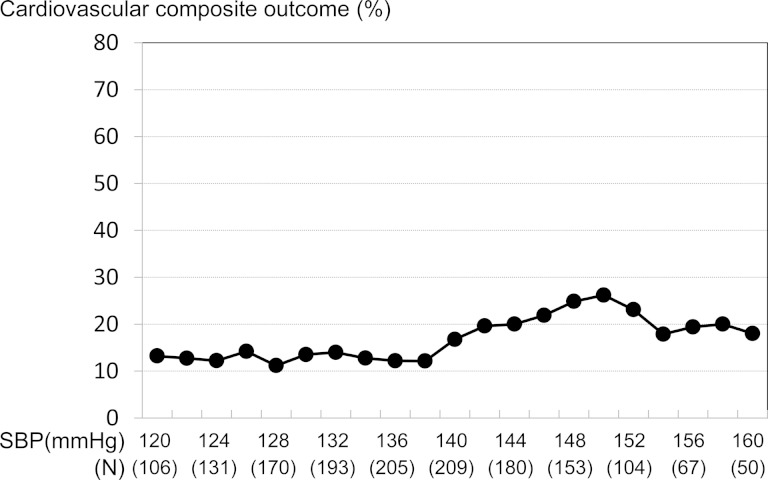
Impacts of SBP on CV outcome
Figure 3 shows the relationship between follow-up SBP and incidence of CV outcome. Follow-up SBP >140 mmHg was associated with high incidence of CV outcome. The HR of CV outcome in the 131–140 mmHg group and >140 mmHg group was 0.81 (0.44–1.48, P = 0.490) and 1.51 (0.91–2.52, P = 0.111) compared with the ≤130 mmHg group. Compared with SBP ≤ 140 mmHg, the HR of SBP > 140 mmHg for CV outcome was 1.69 (1.11–2.56, P = 0.014). The HRs for SBP ≤140 mmHg were similar among patients stratified by UPCR >1 g/gCr with no interaction (Table 4).
FIGURE 3:
Association between CV composite outcome and follow-up SBP. The pattern plot shows the relationship between the percentages of composite CV outcome and follow-up SBP. Each plotted percentage of outcomes is calculated for patients whose follow-up SBP lies within the interval between −6 and +6 mmHg for a specific SBP.
Table 4.
Relationship between follow-up SBP and outcome and modification by baseline UPCR (CV composite outcome)
| n | Follow-up SBP (mmHg) |
P-value for interaction | ||
|---|---|---|---|---|
| ≤140 | >140 | |||
| UPCR | ||||
| <1 g/gCr | 136 | Reference | 1.86 (0.73–4.75) | 0.953 |
| ≥1 g/gCr | 430 | Reference | 1.65 (1.04–2.64) | |
Values are hazard ratio (95% CI).
Impacts of history of CV disease on renal and CV outcomes
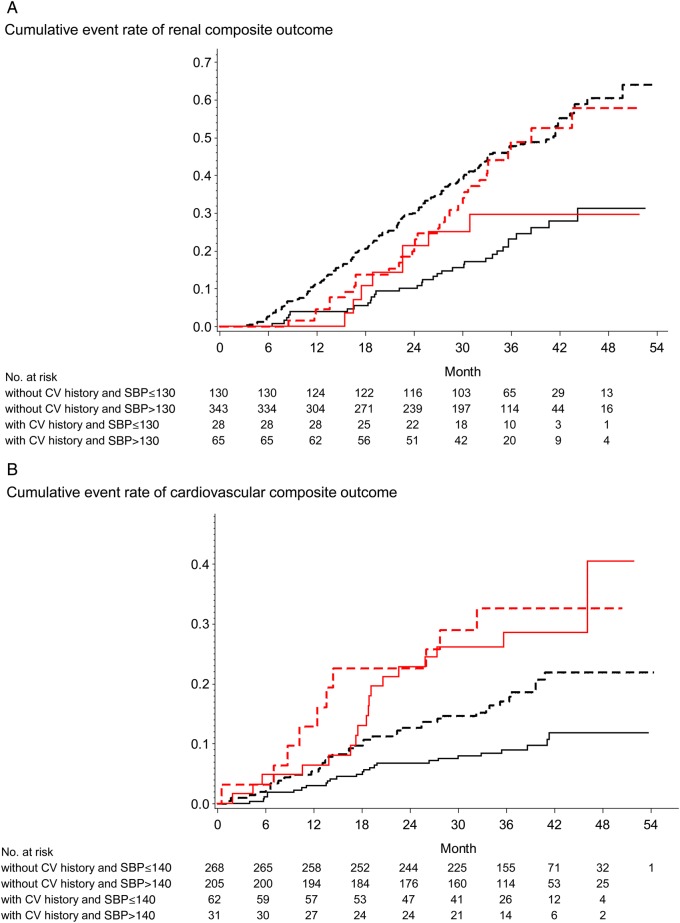
We categorized patients by the presence or absence of history of CV disease and studied the impact of BP on incidence of renal or CV outcomes (Figure 4). The presence of the history of CV disease did not affect the renal outcome (Figure 4A). Compared with patients with SBP ≤ 130 mmHg, the HR for renal outcome for SBP > 130 was 2.64 (1.14–6.09) in those with CV disease and 2.53 (1.73–3.70) in those without CV disease (Table 5). Compared with a referent of SBP ≤ 140 mmHg, SBP > 140 mmHg was associated with increased HR of 2.04 (1.23–3.40, P = 0.006) in patients without CV disease while not in those with history of CV disease with significant interaction (Figure 4B and Table 6).
FIGURE 4:
(A) Effect of follow-up SBP on renal end point in patients without and with history of CVD. (B) Effects of SBP on CV end point in patients without and with history of CVD. The Kaplan–Meier analysis shows time to composite renal and CV outcomes in type 2 diabetic patients with overt proteinuria and renal impairment. All patients were divided into four groups: without CV history and SBP ≤130 (black solid line), without CV history and SBP >130 (black-dashed line), with CV history and SBP ≤130 (red solid line) and with CV history and SBP >130 (red-dashed line) for renal outcome (A); without CV history and SBP ≤140 (black solid line), without CV history and SBP >140 (black-dashed line), with CV history and SBP ≤140 (red solid line) and with CV history and SBP >140 (red-dashed line) for CV outcome (B).
Table 5.
Relationship between follow-up SBP and outcome and modification by history of CV disease (renal composite outcome)
| N | Follow-up SBP (mmHg) |
P-value for interaction | ||
|---|---|---|---|---|
| ≤130 | >130 | |||
| CV history | ||||
| No | 473 | Reference | 2.53 (1.73–3.70) | 0.619 |
| Yes | 93 | Reference | 2.64 (1.14–6.09) | |
Values are hazard ratio (95% CI).
Table 6.
Relationship between follow-up SBP and outcome and modification by history of CV disease (CV composite outcome)
| n | Follow-up SBP (mmHg) |
P-value for interaction | ||
|---|---|---|---|---|
| ≤140 | >140 | |||
| CV history | ||||
| No | 473 | Reference | 2.04 (1.23–3.40) | 0.193* |
| Yes | 93 | Reference | 1.14 (0.51–2.53) | |
Values are hazard ratio (95% CI).
*Significant interaction for a P-value <0.20.
DISCUSSION
In this post hoc analysis of ORIENT involving Asian type 2 diabetic patients with CKD and overt proteinuria, follow-up SBP was linearly associated with increased risk of renal outcomes and rate of decline of renal function as indicated by slope of eGFR. Reduction of SBP to <130 mmHg reduced renal outcome especially in patients with heavy proteinuria (≥1 g/gCr). Although CV outcome was not affected by follow-up SBP in the whole group, a follow-up SBP ≥140 mmHg was associated with increased incidence of CV disease in patients without history of CV diseases. Taken together, these results support the recommendation of reducing SBP <130 mmHg for renoprotection in Asian Type 2 diabetic patients with CKD and overt proteinuria, if tolerated; while reducing SBP <140 mmHg conferred CV benefits only in those without history of cardiovascular disease (CVD). These divergent effects of SBP lowering on CV and renal outcomes call for individualized BP management in patients depending on presence of heavy proteinuria and/or advanced atherosclerosis.
Target BP for renal outcome
In epidemiologic and post hoc analysis, lower BP was linked with lower rates of mortality, CV disease and ESRD in diabetic patients with CKD [4–6]. Only a few randomized controlled trials examined the effects of reducing BP on renal or CV outcome in diabetic patients, mainly in those with normoalbuminuria and/or preserved renal function. In the UKPDS 38 that enrolled newly diagnosed type 2 diabetic patients, a conventional BP target <180/105 mmHg was compared with tight BP target <150/85 mmHg. During a 9-year follow-up period, the mean BP in the intensively and conventionally treated group was 144/82 and 154/87 mmHg, respectively. This difference in achieved BP target was translated to 24% risk reduction in all-diabetes-related end points and 37% risk reduction for microangiopathy [7]. In the ACCORD study that enrolled high-risk patients with long disease duration, the researchers asked whether reducing SBP to <120 mmHg compared with <140 mmHg would further reduce risk for all-cause and CV mortality including stroke. The results showed similar incidence of composite CV outcome between the intensively and conventionally treated group with an achieved SBP of 119 and 133 mmHg, respectively. However, stroke was significantly reduced by intensive BP control [8].
In diabetic nephropathy with overt proteinuria, two post hoc analyses investigated the relationships between BP reduction and incidence of ESRD or CV disease. In a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL), the HR for renal composite outcome or ESRD was similar between patients with achieved SBP <130 mmHg and those with SBP of 130–139 mmHg. However, the HR was increased in patients with SBP ≥140 mmHg [12]. In the Irbesartan Diabetic Nephropathy Trial (IDNT), progressive lowering of SBP toward 120 mmHg improved renal survival, while SBP >149 mmHg was associated with 2.2-fold increased risk for doubling of SCr or ESRD compared with SBP <134 mmHg. These latter results agreed with ours where the lowest risk of renal outcome was found in patients with SBP ≤130 mmHg, albeit these benefits were not found in patients with prior history of CVD [20]. In another Japanese study, achieving a target SBP <130 mmHg did not improve renal outcome in patients with silent cerebrovascular ischemia in type 2 diabetes [21]. These results suggested that preexisting atherosclerotic diseases might modify the effects of BP lowering on renal outcomes.
Baseline proteinuria and BP for renal outcome
In this post hoc analysis, patients with baseline proteinuria <1.0 g/gCr had a low incidence of renal outcomes regardless of baseline BP. In the MDRD study that enrolled patients without diabetes, intensive BP control targeting <125/75 mmHg (achieved mean BP of 126/75 mmHg) did not confer renoprotection compared with conventional BP control targeting at 140/90 mmHg (achieved mean BP of 134/80 mmHg). However, in patients with baseline proteinuria ≥3.0 g/gCr, intensive BP control improved renal outcome [22].
In a meta-analysis of 11 randomized controlled trials involving nondiabetic patients with baseline proteinuria <1.0 g/gCr, the risk for doubling of SCr or ESRD was similar within an SBP range of 110–160 mmHg [23]. In our study, among patients with baseline proteinuria <1.0 g/gCr, the risk of renal outcomes was similar within a SBP range of 120–140 mmHg (Figure 2). In contrast, in patients with heavy proteinuria (≥1.0 g/gCr), reduction in SBP was associated with further reduction in renal outcomes, highlighting the interactive effects between SBP and proteinuria on renal outcomes. To this end, proteinuria may play a causal role in progressive renal disease by damaging podocytes, activating inflammation and worsening cardiometabolic risk factors [24, 25]. Thus, in these high-risk patients, intensive SBP lowering might break these vicious cycles.
Target SBP for CV outcome
Compared with stroke and renal disease, the optimal level of SBP for CV outcomes in type 2 diabetes is even more controversial. In the ACCORD study, intensive BP lowering to 120 mmHg reduced risk of stroke by 41% but did not reduce risk of myocardial infarction and CV mortality compared with conventional BP control [8]. In a meta-analysis of 13 randomized controlled trials involving type 2 diabetic patients, intensive SBP control to <135 mmHg reduced risk for all-cause mortality by 10% and stroke by 17% with no additional benefit on CV or renal outcomes but increased the risk of serious adverse events by 20%, compared with a conventional SBP target of 140 mmHg. Further lowering of SBP to 130 mmHg reduced risk of stroke by 40% but increased risk of adverse events by 40% [1].
In the IDNT study, progressive lowering of SBP to 120 mmHg was associated with improved renal outcome and patient survival. However, below this threshold, the risk of all-cause mortality increased supporting an optimal SBP target of 120–130 mmHg in patients with type 2 diabetes and overt proteinuria [18]. In a post hoc analysis combining data from RENAAL and IDNT, there was a tendency for a higher CV risk in patients with an average SBP of 120 mmHg at 6 months than those with SBP of 140 mmHg [26]. In the VA-NEPHRON-D study that examined the combination therapy of losartan and lisinopril versus losartan monotherapy in 1448 type 2 diabetic patients with CKD and proteinuria, the HR of secondary CV outcome was 0.78, albeit insignificant. However, the study was prematurely stopped due to increased risk of hyperkalemia and adverse renal events [27]. This series of analyses has led to the recommendation of a target SBP <140 mmHg in patients with type 2 diabetes and cautious use of dual blockade treatment of the renin-angiotensin system.
However, in Asian type 2 diabetic patients with overt nephropathy, our data suggested that an SBP <140 mmHg improved CV outcomes and SBP ≤130 mmHg reduced renal outcome and decline of renal function. In this closely monitored clinical trial where 70% of patients received concomitant treatment with angiotensin-converting enzyme (ACE) inhibitor, despite the higher risk of hyperkalemia, there was no increased risk of acute renal events in patients treated with both olmesartan and ACE inhibitor [15]. In a recent systematic review of BP-lowering trials in type 2 diabetes, the authors concluded that every 10 mmHg lowering of SBP was associated with an HR of 0.71 and 0.86 for incident and worsening albuminuria in patients with a baseline SBP ≥130 and <130 mmHg, respectively. They further highlighted that more clinical details and long-term studies were needed given the heterogeneity of the clinical profile of these patients [28]. In this light, given the high risk for stroke and renal disease [14] as well as the importance of CKD in predisposing CV disease in Asian populations [29], a target SBP of 130 mmHg might confer renoprotection in Asian type 2 diabetic patients with overt nephropathy.
Limitations
CKD is a heterogeneous condition due to multiple causes including but not limited to metabolic, ethnic, genetic, inflammatory, vascular and growth factors [30]. Compared with nondiabetic patients, those with diabetes often harbor multiple risk factors that contribute to their high risk for CKD. In Asian type 2 diabetic patients, inflammation and genetic factors [14, 31] play important pathogenetic roles in whom optimal control of BP and blockade of the renin angiotensin system may have particular benefits [32, 33]. Besides, Asian diabetic patients are less prone to develop CV disease, except for stroke, and more likely to develop renal disease compared with their Caucasian counterparts [33, 34]. In the ORIENT, 245 and 93 patients developed renal and CV outcomes, respectively, a near 3-fold difference. Thus, the lack of association between SBP and CV outcomes might be due to sample size or ethnicity. Except for a few studies like IDNT, IRMA and RENAAL [35–37], many randomized controlled trails that examined the effects of angiotensin II inhibition on renal outcome recruited heterogeneous populations with both diabetic and nondiabetic subjects such as ONTARGET [38] or diabetic patients with normoalbuminuria with low risk for CKD, like ROADMAP [39]. In contrast, participants of the ORIENT were relatively homogenous of Asian ethnicity with heavy proteinuria and renal impairment.
CONCLUSION
In Asian type 2 diabetic patients with overt nephropathy, the presence of proteinuria more than 1g/gCr and prior history of CV disease modified the effects of achieved SBP on CV and renal outcomes. While a mean SBP ≤130 mmHg conferred renoprotection especially in patients with heavy proteinuria, a mean SBP ≤ 140 mmHg conferred CV protection especially in those without history of CV disease. Given the phenotypic heterogeneity of diabetes and its complications, most international guidelines call for individualized rather than ‘one size fits all’ treatment targets for hyperglycemia [40], although the recommendation is less clear in BP management. Pending definitive evidence, physicians might consider reducing SBP to 130 mmHg for renoprotection although caution should be exercised in those with advanced atherosclerosis.
CONFLICT OF INTEREST STATEMENT
E.I. received consultancy and lecture fees from Daiichi Sankyo, Kyowa-Hakko-Kirin, Kaken. S.I. received consulting and lecture fees and grant support from Daiichi-Sankyo. A.H., F.K. and T.Y. are employees of Daiichi-Sankyo, and F.K. contributed to the analysis of the data. M.H., H.M. and J.C.N.C. received consulting and lecture fees and grant support from Daiichi-Sankyo.
ACKNOWLEDGEMENT
The ORIENT study was supported by a research grant from Daiichi Sankyo.
REFERENCES
- 1.Bangalore S, Kumar S, Lobach I et al. . Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation 2011; 123: 2799–2810 [DOI] [PubMed] [Google Scholar]
- 2.Khouri Y, Steigerwalt SP, Alsamara M et al. . What is the ideal blood pressure goal for patients with stage III or higher chronic kidney disease? Curr Cardiol Rep 2011; 13: 492–501 [DOI] [PubMed] [Google Scholar]
- 3.Allen M, Kelly K, Fleming K. Uncertainty about the systolic blood pressure target in people with diabetes. Can Fam Physician 2013; 59: 128–131 [PMC free article] [PubMed] [Google Scholar]
- 4.Lewington S, Clarke R, Qizilbash N et al. . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913 [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC et al. . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108: 2154–2169 [DOI] [PubMed] [Google Scholar]
- 6.Lawes CM, Rodgers A, Bennett DA et al. . Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21: 707–716 [DOI] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713 [PMC free article] [PubMed] [Google Scholar]
- 8. ACCORD Study Group, Cushuman WC, Evans GW, Byington RP et al. . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association. Standards for medical care in diabetes—2013. Diabetes Care 2013; 36: S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2: 337–414 [Google Scholar]
- 11.James PA, Oparil S, Carter BL et al. . 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–520 [DOI] [PubMed] [Google Scholar]
- 12.Bakris GL, Weir MR, Shanifar S et al. . RENAAL Study Group: effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med 2003; 163: 1555–1565 [DOI] [PubMed] [Google Scholar]
- 13.Berl T, Hunsicker LG, Lewis JB et al. . Collaborative Study Group. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005; 16: 2170–2179 [DOI] [PubMed] [Google Scholar]
- 14.Kong AP, Xu G, Brown N et al. . Diabetes and its comorbidities—where east meets west. Nat Rev Endocrinol 2013; 9: 537–547 [DOI] [PubMed] [Google Scholar]
- 15.Imai E, Chan JC, Ito S et al. . Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 2011; 54: 2978–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenland S. Introduction to regression models. In: Rothman KJ, Greenland S, Lash TL (eds). Modern Epidemiology. 3rd edn Philadelphia: Lippincott-Williams-Wilkins, 2008, pp. 381–417 [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481 [Google Scholar]
- 18.Mastuo S, Imai E, Horio M et al. . Revised equations for estimating glomerular filtration rate (GFR) from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 19.Stevens LA, Coresh J, Greene T et al. . Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 2006; 354: 2473–2483 [DOI] [PubMed] [Google Scholar]
- 20.Pohl MA, Blumenthal S, Cordonnier DJ et al. . Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16: 3027–3037 [DOI] [PubMed] [Google Scholar]
- 21.Uzu T, Kida Y, Yamauchi A et al. . The effects of blood pressure control levels on the renoprotection of type 2 diabetic patients without overt proteinuria. J Am Soc Hypertens 2012; 6: 124–131 [DOI] [PubMed] [Google Scholar]
- 22.Klahr S, Levey AS, Beck GJ et al. . The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330: 877–884 [DOI] [PubMed] [Google Scholar]
- 23.Jafar TH, Stark PC, Schmid CH et al. . Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139: 244–252 [DOI] [PubMed] [Google Scholar]
- 24.Koop K, Eikmans M, Wehland M et al. . Selective loss of podoplanin protein expression accompanies proteinuria and precedes alterations in podocyte morphology in a spontaneous proteinuric rat model. Am J Pathol 2008; 173: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Brozovic S, Xu J et al. . Inflammatory gene expression in overt diabetic kidney during the development of nephropathy. Nephron Exp Nephrol 2011; 119: e8–e20 [DOI] [PubMed] [Google Scholar]
- 26.Holtkamp FA, de Zeeuw D, de Graeff PA et al. . Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J 2011; 32: 1493–1499 [DOI] [PubMed] [Google Scholar]
- 27.Fried LF, Emanuele N, Zhang JH et al. . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369: 1892–1903 [DOI] [PubMed] [Google Scholar]
- 28.Emdin CA, Rahimi K, Neal B et al. . Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313: 603–615 [DOI] [PubMed] [Google Scholar]
- 29.Nakayama M, Metoki H, Terawaki H et al. . Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population—the Ohasama study. Nephrol Dial Transplant 2007; 22: 1910–1915 [DOI] [PubMed] [Google Scholar]
- 30.Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev 2004; 25: 971–1010 [DOI] [PubMed] [Google Scholar]
- 31.Mooyaart AL, Valk EJ, van Es LA et al. . Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia 2011; 54: 544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrish NJ, Wang SL, Stevens LK et al. . Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001; 44 (Suppl 2): S14–S21 [DOI] [PubMed] [Google Scholar]
- 33.Chan JC, Wat NM, So WY et al. . Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL Study. Diabetes Care 2004; 27: 874–879 [DOI] [PubMed] [Google Scholar]
- 34.Kurokawa K, Chan JC, Cooper ME et al. . Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes: a subanalysis of Japanese patients from the RENAAL Study. Clin Exp Nephrol 2006; 10: 193–200 [DOI] [PubMed] [Google Scholar]
- 35.Brenner BM, Cooper ME, de Zeeuw D et al. . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 36.Parving HH, Lehnert H, Brochner-Mortensen J et al. . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878 [DOI] [PubMed] [Google Scholar]
- 37.Lewis EJ, Hunsicker LG, Clarke WR et al. . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 38.Yusuf S, Teo KK, Pogue J et al. . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559 [DOI] [PubMed] [Google Scholar]
- 39.Haller H, Ito S, Izzo JL Jr et al. . Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011; 364: 907–917 [DOI] [PubMed] [Google Scholar]
- 40.Raz I, Riddle MC, Rosenstock J et al. . Personalized management of hyperglycemia in type 2 diabetes: reflections from a diabetes care editors’ expert forum. Diabetes Care 2013; 36: 1779–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]