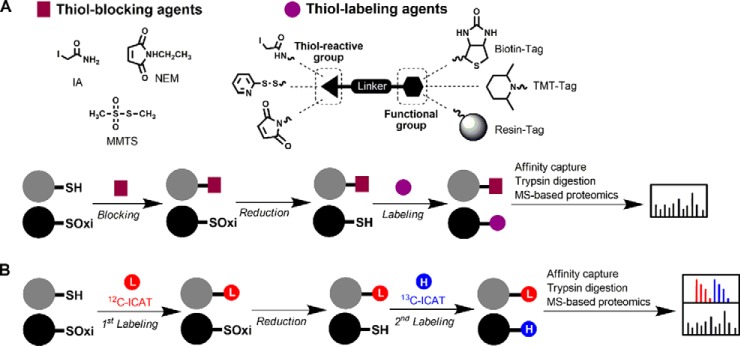
Fig. 3.
Biotin-switch techniques (BST) and variations for the proteome-wide identification and quantification of thiol redox modifications. A, BST involve differential thiol labeling of reduced and oxidized thiols. Free thiols are first blocked with a small-molecule thiol-reactive agent such as iodoacetamide (IA), N-ethylmaleimide (NEM), or methyl methanethiosulfonate (MMTS). Reversibly oxidized thiols are reduced with a specific reducing agent, labeled with another thiol-reactive agent with a functionalized group, such as biotin, TMT, or resin, enriched by affinity purification or resin-assisted capture, and analyzed by MS-based proteomics. B, OxICAT is used to measure the relative content of oxidized thiols in thiol proteome. Free thiols are first labeled with the light (12C) ICAT (red), while oxidized proteins in the same sample are subsequently reduced and labeled with the heavy (13C) ICAT (blue). The ICAT labeled sample is then subjected to trypsin digestion and affinity purification. The resulting peptides are detected and quantified by MS-based proteomics. The extent of thiol oxidation in any given peptide is determined by the ratios of heavy to light precursor ion signal intensity.