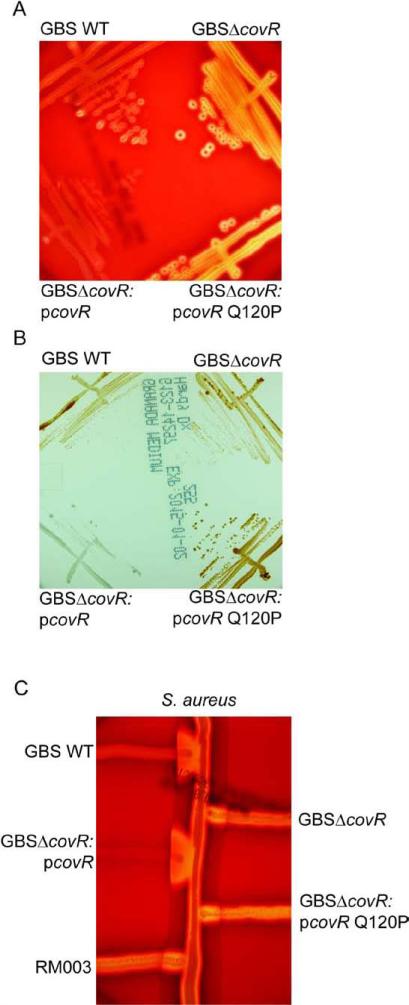
Figure 3. The Q120P amino acid substitution in CovR abolished complementation of a GBSΔcovR mutant.
(A) The zone of lysis around the colonies on sheep blood agar represents hemolytic activity. Note that hemolytic activity is increased in GBSΔcovR and introduction of the plasmid encoding WT CovR (pCovR) into GBSΔcovR decreased hemolytic activity similar to WT GBS whereas at the pCovR plasmid with the Q120P (pCovRQ120P) substitution did not.
(B) WT, mutant and complemented strains on Granada Media. Note that complementation of GBSΔcovR with WT pCovR plasmid substantially decreased pigment expression whereas the pCovR plasmid with the Q120P (pCovRQ120P) substitution did not.
(C) CAMP factor activity is denoted by the triangular zone of lysis at the junction between GBS and a β-lysin producing strain of S. aureus. Note that complementation of GBSΔcovR with WT pCovR plasmid restored CAMP factor expression to levels observed in WT GBS whereas the pCovR plasmid with the Q120P (pCovRQ120P) substitution did not.