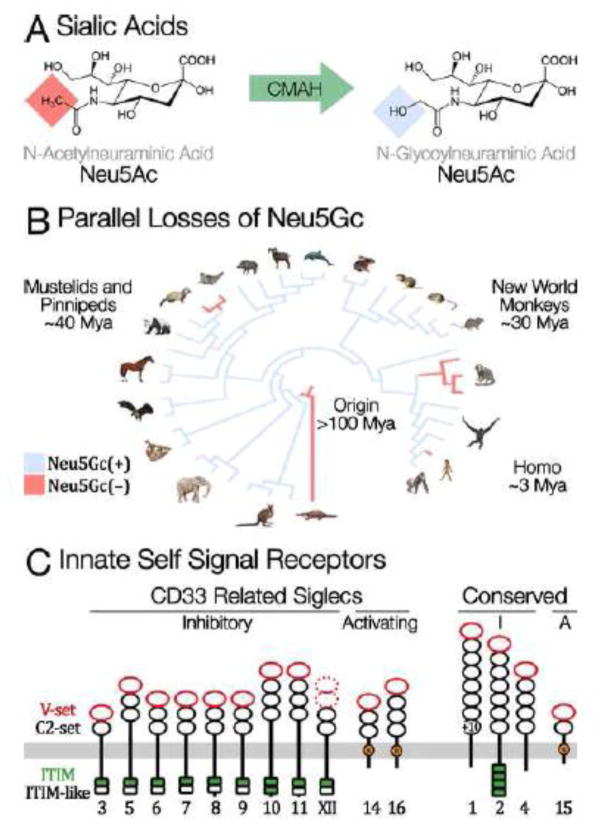
Figure 3. Sialic Acids: Parallel Evolution of Innate Self-Signals.
A) Sialic acids are nine-carbon monosaccharides found at the terminal tips of N-glycans, on glycolipids, and as long chains of polysialic acid. N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) are two of the most common forms: each a family of molecules with various modifications of the canonical monosaccharide. Neu5Gc is synthesized from Neu5Ac by the CMAH protein (cytidine monophosphate-N-acetylneuraminic acid hydroxylase), which hydroxylates a terminal methyl group. Neu5Gc is a private glycan in the sense that no pathogen has yet been found to synthesize this monosaccharide. B) Humans cannot synthesize Neu5Gc, because human CMAH was inactivated over two million years ago [68]. The inactivating mutation fixed rapidly after originating, which suggests that the loss could have been adaptive – driven by pathogen avoidance, sexual conflict, or a combination of the two [69]. Independent losses of CMAH have recently been found in New World Primates [62] and in Mustelids [70]. C) Sialic acids are bound by a family of Siglec receptor proteins expressed on most immune cells [71]. Binding generally inhibits inflammation, thus sialic acids are innate markers of self. Many pathogens bind Siglecs to exploit these immunosuppressive effects, and in response several immune-activating Siglecs have evolved. Other pathogens use Neu5Gc itself as a receptor on host cells. Several human pathogens have modified their receptor affinity to recognize Neu5Ac following the loss of Neu5Gc in humans.