Abstract
Purpose
Testis sparing surgery (TSS) is a well-known technique in the treatment of small testicular masses. Grayscale ultrasound (US), color/power Doppler US (CPDUS) and contrast-enhanced ultrasound (CEUS) are considered the best diagnostic imaging tools in those patients. Aim of this study was to assess the role of US imaging in the detection of small testicular masses in monorchid patients after orchiectomy for malignant neoplasm, and in guiding surgery to reach the target and also to differentiate lesions which presented vascular activity within the mass.
Methods
From January 2011 to October 2014, 18 patients were enrolled in this study. They had previously undergone orchiectomy and were investigated for suspected contralateral disease. During routine follow-up, all patients underwent grayscale US. If findings were positive, CPDUS and CEUS were performed and eventually all patients underwent surgery. After exteriorization of the testis, the small mass was identified by intraoperative US, and a needle was placed under US guidance. After excision of the mass, frozen section examination was performed. When malignancy was found, radical orchiectomy was performed; if histological outcome was negative, the healthy testis was conserved.
Results
All patients underwent grayscale US examination, which showed small hypoechoic masses. Each mass identified at US imaging was confirmed at surgery. All patients underwent CPDUS; 12/19 lesions showed blood flow while 7/19 showed absence of blood flow. At CEUS, 16/19 lesions showed enhancement and subsequent histological examination revealed that 8 were seminomas and 3 were Leydig cell tumors. In 5/19 cases CEUS showed the presence of lesions (focal inflammatory lesions) and in 3/19 cases CEUS was negative.
Conclusions
TSS in monorchid patients may be a safe procedure leading to excellent results. We therefore consider it a valid alternative to radical orchiectomy, and US imaging is essential to guide the resection of non-palpable neoplasms and to exclude concomitant lesions.
Keywords: Ultrasound, Color-doppler, Testicular neoplasm, CEUS, Monorchid
Riassunto
Scopo
La testis sparing surgery (TSS) è una tecnica ben nota nel trattamento di piccole masse testicolari. L’ecografia in scala di grigi (US), il color/power Doppler (CPDUS) e l’ecografia con mezzo di contrasto (CEUS) sono considerati i migliori strumenti di diagnostica per immagini in questi pazienti. Scopo di questo studio è stato quello di valutare il ruolo dell’imaging ecografica nell’individuazione delle piccole masse testicolari nei pazienti monorchidi dopo orchiectomia per neoplasia maligna, nel ruolo di guida durante l’intervento chirurgico e nella differenziazione delle lesioni che presentavano attività vascolare dentro la massa.
Metodi
Da Gennaio 2011 a Novembre 2014, 18 pazienti (19 lesioni), orchiectomizzati per neoplasia, durante il follow-up di routine, sono stati sottoposti a ecografia. Se positivi, venivano sottoposti a CPDUS e CEUS ed ad intervento chirurgico. La piccola massa, era identificata e sotto guida ecografica marcata con ago. Dopo l’asportazione della massa, è stato eseguito l’esame istologico estemporaneo. In caso di malignità, veniva eseguita una orchiectomia radicale; se l’esito istologico era negativo, il testicolo sano veniva conservato.
Risultati
In tutti i pazienti l’ecografia ha mostrato 19 piccole masse ipoecogene confermate all’intervento chirurgico. Al CPDUS; 12/19 hanno mostrato presenza di flusso ematico, 7 hanno mostrato assenza di segnale. Alla CEUS, 16/19 sono risultate positive e l’esame istologico ha dimostrato 8 seminomi, 3 tumori a cellule di Leydig. In 5 casi la CEUS ha mostrato la presenza di lesioni (lesioni infiammatorie focali) e in 3 è stata negativa.
Conclusioni
La TSS nei pazienti monorchidi può essere una procedura sicura che porta a eccellenti risultati. Tale tecnica può essere una valida alternativa all’orchiectomia radicale. L’imaging ecografico è essenziale per il riconoscimento e la caratterizzazione della lesione, per guidare la resezione delle neoplasie non palpabili e per escludere lesioni concomitanti.
Introduction
Testis sparing surgery (TSS) is a well-known technique in the treatment of small testicular masses [1, 2]. Some investigators also perform TSS to treat germ cell tumors (GCTs) in the presence of a normal, functioning contralateral testis as 70 % of GCTs at histological examination appeared benign [3]. The objective of this approach is to reduce psychosocial consequences linked to radical orchiectomy and to save endocrinal function. Innovative surgical techniques using a surgical microscope and knowledge acquired during surgery for infertility treatment increase the efficacy of the operation and the possibility of sparing the healthy tissue [4, 5].
After orchiectomy for malignant disease, most recurrences occur in the contralateral testis within the first 2 years after curative therapy. Frequent and intensive follow-up is therefore essential during this period and should include physical examination and monitoring of tumor markers as well as US pattern every 3 months [6]. Grayscale ultrasound (US), color/power Doppler US (CPDUS) and contrast-enhanced ultrasound (CEUS) are considered the best diagnostic imaging tools in these patients [7].
Aim of this study was to assess the role of US imaging in the detection of small testicular masses in monorchid patients after orchiectomy for malignant neoplasm, and in guiding surgery to reach the target and also to differentiate lesions which presented vascular activity within the mass.
Materials and methods
From January 2011 to October 2014, 18 patients (mean age 31; age range 25–35) were enrolled in this study. They had previously undergone orchiectomy and were investigated for suspected contralateral disease. Inclusion criteria were: monorchid patients with a small intra-testicular mass, hypoechoic at grayscale US (max diameter 1.5 cm).
During routine follow-up after therapy, all patients underwent grayscale US. In case of positive findings, they underwent CPDUS and CEUS and finally all patients underwent surgery.
All examinations were performed on Technos (Esaote Biomedica, Italy), Aloka (Hitachi, Japan) and Aplio (Toshiba, Japan) using linear 7.5 MHz probes. CPDUS was performed with scanning parameters set for maximum sensitivity to flow velocities at the different levels and power output was increased to maximum. Color gain was increased until just prior to the appearance of random noise. Pulse repetition frequency was set at the lowest possible level. Multiple axial, longitudinal and oblique scans were obtained.
All patients subsequently underwent CEUS using a low mechanical index (range 0.04–0.1) after administration of contrast agent (SonoVue, Bracco™). A total of 4.8 ml was administered in two intravenous bolus doses of 2.4 ml; the second dose was injected 15 min after the first, and both were followed by 5 ml of saline flush.
Evaluation criteria were the following: lesion identification, detection of vascularization, presence of CEUS enhancement.
All 18 patients underwent TSS after preoperative evaluation based on clinical examination and biomarkers. US imaging parameters were lesion size, echogenicity, vascularization detected by CPDUS and presence of an enhancing mass at CEUS.
All patients underwent surgical exploration of the testis by inguinal access. After exteriorization of the testis, the small mass was identified by intraoperative US, and under US guidance a needle was placed. After excision of the mass, frozen section examination (FSE) was performed in all patients in addition to multiple biopsies of the surrounding tissue. When malignancy was found, radical orchiectomy was performed; if histological outcome was negative, the healthy testis was conserved.
Results
The 18 testicles presented a total of 19 lesions, as one testicle presented two similar, closely located lesions, which were removed by a single excision. All lesions were hypoechoic at grayscale US, and mean dimension was 0.8 cm (range 0.5–1.5 cm). Histological examination identified 3 Leydig cell tumors, 8 focal inflammatory lesions and 8 seminomas.
All patients underwent grayscale US examination, which showed small hypoechoic masses. Each mass identified at US imaging was confirmed at surgery. All patients underwent CPDUS; 12/19 lesions showed blood flow while 7/19 showed absence of blood flow.
At CEUS, 16/19 lesions showed enhancement; 11/19 were strongly enhanced and histological examination showed that 8 were seminomas and 3 were Leydig cell tumors. In 5/19 cases CEUS showed the presence of lesions (focal inflammatory lesions) and in 3/19 cases CEUS was negative.
Discussion
Improved resolution of US machines has led to an increase in detection of small testicular masses, which may be in an early stage of malignant disease or benign tumors. Recent studies show that approximately, 80 % of non-palpable masses are benign [8].
Organ sparing surgery is widely accepted for treating benign lesions, and TSS is considered also for treating malignant diseases of the testis, thereby giving credit to the opinion that radical orchiectomy is not the only option in the management of testicular carcinoma [9].
TSS is a widely used technique for the treatment of small testicular masses, and the objective of this technique is to save the endocrinal function especially in monorchid patients [10].
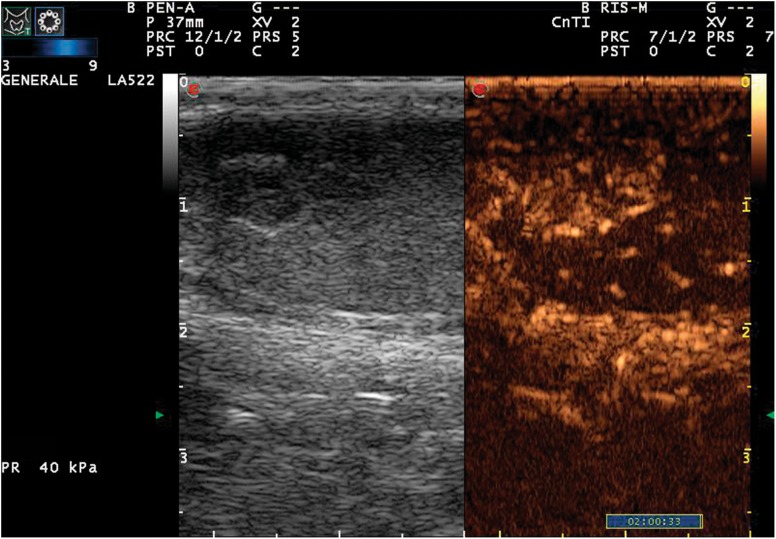
Grayscale US, CPDUS and CEUS are essential methods for identifying and characterizing small testicular masses, and surgery is currently the only effective option for treating cancer [11, 12]. In our study, 19/19 lesions were identified at grayscale US imaging. CPDUS showed vascularization in 11/19 lesions, which were all malignant (8 seminomas and 3 Leydig cell tumors) (Fig. 1). The lesions that did not present vascularization at CPDUS were very small (minor than 0.5 cm), and histological examination identified them as inflammatory lesions. This shows that the sensitivity of CPDUS is high in the study of slow blood flow and small lesions.
Fig. 1.
Color-Doppler signal acquisition; longitudinal scan of the testis, note the small dimension of lesion, still presence of vascular signs within the lesion is visible
CEUS showed vascularization in 5/19 lesions thereby increasing the specificity of the method. Three lesions were negative at CEUS, but they were linked to inflammatory disease. The results obtained in this study show that CEUS enhancement indicates the presence of an active lesion, which requires histological examination to be accurately characterized (Fig. 2).
Fig. 2.
Qualitative CEUS imaging. Longitudinal scan 30 s after first injection, presence of strong enhancement within the lesion
Grayscale US imaging can readily detect testicular lesions. The sensitivity of CPDUS in differentiating malignant from inflammatory lesions is elevated, but specificity is moderate. However, CEUS improves this specificity. Further studies of CEUS performance are required to confirm the ability of this method to differentiate benign from malignant lesions.
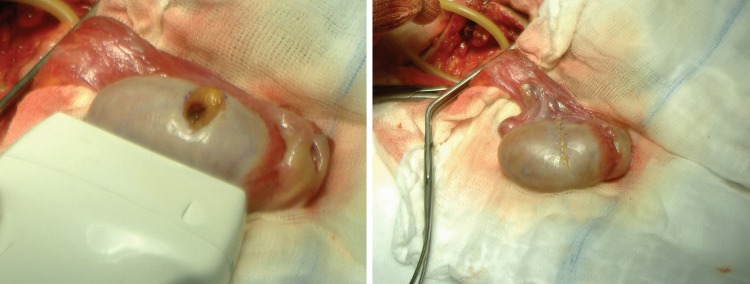
In this study, the role of US imaging was not only to identify the presence of lesions and to attempt an accurate characterization, but also to provide US guidance during partial resection. This study confirmed that it is possible to identify a mass and mark it with a needle under US guidance (Fig. 3).
Fig. 3.
To mark the lesion (a), a needle is placed under US guidance; the tip of the needle is positioned inside the mass (b)
To save the contralateral testis and help preserve an acceptable quality of life, the described procedure is currently the most adequate solution thanks to intraoperative US imaging and fast extemporaneous histological examination (Fig. 4). US imaging is helpful in guiding the surgeon to the appropriate biopsy sites and subsequently for CPDUS evaluation of possible residual malignant tissue after surgical removal of the mass.
Fig. 4.
The lesion has been removed and the testis is reinserted into the scrotum
Conclusion
Our experience shows that TSS performed for small testicular masses in monorchid patients may be a safe procedure leading to excellent results. We therefore consider it a valid alternative to radical orchiectomy, and US imaging is essential to guide the resection of non-palpable neoplasms and to exclude concomitant lesions.
Conflict of interest
All the above authors declare no conflict of interest at all.
Ethical standard
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the Helsinki Declaration of 1975 and its late amendments.
References
- 1.Buckspan MB, Klotz PG, Goldfinger M, et al. Intraoperative ultrasound in the conservative resection of testicular neoplasms. J Urol. 1989;141:326–327. doi: 10.1016/s0022-5347(17)40756-7. [DOI] [PubMed] [Google Scholar]
- 2.Giannarini G, Mogorovich A, Bardelli I, et al. Testis-sparing surgery for benign and malignant tumors: a critical analysis of the literature. Indian J Urol. 2008;24:467–474. doi: 10.4103/0970-1591.44249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich A, Weissbach L, Höltl W, et al. Organ: German Testicular Cancer Study Group sparing surgery for malignant germ cell tumor of the testis. J Urol. 2001;166:2161–2165. doi: 10.1016/S0022-5347(05)65526-7. [DOI] [PubMed] [Google Scholar]
- 4.Eifler JB, Jr, King P, Schlegel PN. Incidental testicular lesions found during infertility evaluation are usually benign and may be managed conservatively. J Urol. 2008;180:261–264. doi: 10.1016/j.juro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Steiner H, Höltl L, Maneschg C, et al. Frozen section analysis guided organ-sparing approach in testicular tumors: technique, feasibility, and long-term results. Urology. 2003;62:508–513. doi: 10.1016/S0090-4295(03)00465-5. [DOI] [PubMed] [Google Scholar]
- 6.Albers P, Albrecht W, Algaba F. Guidelines on testicular cancer. Arnhem: European Association of Urology; 2013. p. 2528. [Google Scholar]
- 7.Shah A, Lung PF, Clarke JL, et al. Re: new ultrasound techniques for imaging of the indeterminate testicular lesion may avoid surgery completely. Clin Radiol. 2010;65:496–497. doi: 10.1016/j.crad.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Carmignani L, Gadda F, Gazzano G, et al. High incidence of benign testicular neoplasm diagnosed by ultrasound. J Urol. 2003;170:1783–1786. doi: 10.1097/01.ju.0000092066.01699.90. [DOI] [PubMed] [Google Scholar]
- 9.Giannarini G, Dieckmann KP, Albers P, et al. Organ-sparing surgery for adult testicular tumours: a systematic review of the literature. Eur Urol. 2010;57:780–790. doi: 10.1016/j.eururo.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 10.De Stefani S, Isgrò G, Varca V, et al. Microsurgical testis-sparing surgery in small testicular masses: 7 years retrospective management and results. Urology. 2012;79:858–862. doi: 10.1016/j.urology.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Powell TM, Tarter TH. Management of nonpalpable incidental testicular masses. J Urol. 2006;176:96. doi: 10.1016/S0022-5347(06)00496-4. [DOI] [PubMed] [Google Scholar]
- 12.Assaf GJ. Non-palpable testicular lesion: the case for testicular preservation. Can J Urol. 2006;13:3034. [PubMed] [Google Scholar]