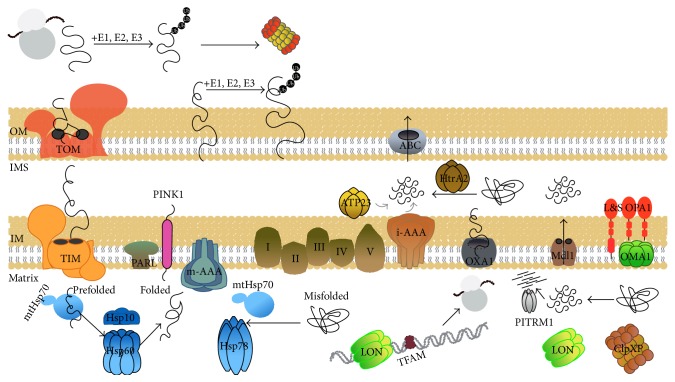
Figure 1.
Mitochondrial quality control by molecular chaperones and proteases. Mitochondrial precursors synthesized in cytosol are imported in the mitochondrial matrix via the TOM and TIM translocases. Misfolded precursors are degraded by the 26S proteasome in the cytosol before they enter mitochondria; the 26S proteasome also degrades (following ubiquitination) proteins of the outer mitochondria membrane (OM). Precursors imported in the mitochondrial matrix are bound to chaperones (e.g., mtHsp70 and Hsp60/Hsp10) which then drive their proper folding; mtHsp70 along with Hsp78 also promote protein disaggregation during stress conditions. The polypeptides of the respiratory complex protein machines which are encoded by either mtDNA or genomic DNA are transported into the inner membrane (IM) by the Oxa1 peptide transporter. Damaged and/or unfolded matrix proteins are degraded by the LON, ClpXP, and m-AAA proteases, while the generated peptides can be further degraded by PITRM1; LON protease also degrades the TFAM transcription factor. Peptides generated by the ClpXP protein are transported across the inner mitochondrial membrane by the matrix ATP-dependent peptide transporter HAF-1 (Mdl1 in yeast). The PINK1 protein is encoded at the genomic DNA and after being transported at the IM it is processed by PARL. In the case of mitochondrial dysfunction or damage PINK1 translocates at the OM and facilitates the activation of autophagy/mitophagy machinery (see text). Similarly to PINK1, OPA1 is imported from the cytosol and is processed in long (L) and short (S) isoforms which are located at the IM and the intermembrane space (IMS), respectively. During mitochondrial dysfunction OPA1 isoforms are processed by OMA1 (and, likely, PARL), while unfolded, misfolded, and/or damaged proteins of the IMS are processed by the HtrA2 and i-AAA proteases; generated peptides are then released in the cytosol by the ATP binding cassette transporter (ABC transporter). Mitochondrial inner membrane protease ATP23 is thought to participate in the maintenance of the respiratory chain; however, its role still remains to be elucidated. Mentioned molecules along with their relative topologies and processing (arrows) are indicated in the figure.