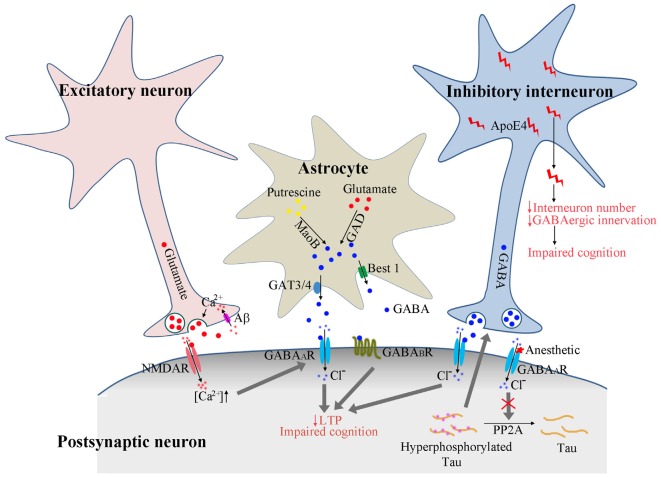
Figure 1.
Proposed model of GABAergic signaling in AD pathogenesis. Calcium enters the presynaptic terminal via Aβ formed pores on cell membrane. The increased calcium concentration triggers presynaptic glutamate neurotransmitter release and activates postsynaptic receptors. The activated NMDA receptors further enhance the GABAA receptor activation to dampen the overexcitation. In astrocytes, GABA could be synthesized from putresine or glutamate and released via GAT3/4 or Best1 channel. The release of GABA from astrocytes may be enhanced under AD conditions to activate the extrasynaptic GABAA and GABAB receptors, resulting in suppressed long-term potentiation (LTP) and impaired cognition. The activation of GABAA receptors by anesthetics reduces PP2A binding with tau protein, resulting in tau hyperphosphorylation, which feeds back and enhances the activation of GABAergic interneurons and GABA release. ApoE4 secreted from GABAergic interneurons results in reduced interneuron number and reduced GABAergic innervation to other neurons, eventually leading to the disruption of neuronal circuitry and impaired cognition.