Abstract
The state-of-the-art for personal sampling for inhalable aerosol hazards is constrained by issues of sampler cost and complexity; these issues have limited the adoption and use of some samplers by practicing hygienists. Thus, despite the known health effects of inhalable aerosol hazards, personal exposures are routinely assessed for only a small fraction of the at-risk workforce. To address the limitations of current technologies for inhalable aerosol sampling, a disposable inhalable aerosol sampler was developed and evaluated in the laboratory. The new sampler is designed to be less expensive and simpler to use than existing technologies. The sampler incorporates a lightweight internal capsule fused to the sampling filter. This capsule-filter assembly allows for the inclusion of particles deposited on the internal walls and inlet, thus minimizing the need to wash or wipe the interior sampling cassette when conducting gravimetric analyses. Sampling efficiency and wall losses were tested in a low-velocity wind tunnel with particles ranging from 9.5 to 89.5 μm. The results were compared to the proposed low-velocity inhalability criterion as well as published data on the IOM sampler. Filter weight stability and time-to-equilibrium were evaluated as these factors affect the practicality of a design. Preliminary testing of the new sampler showed good agreement with both the IOM and the proposed low-velocity inhalability curve. The capsule and filter assemblies reached equilibrium within 25h of manufacturing when conditioned at elevated temperatures. After reaching equilibrium, the capsule-filter assemblies were stable within 0.01mg.
KEYWORDS: air pollution, exposure, low cost, particulate matter, sampling efficiency, wall deposits
INTRODUCTION
The burden of disease from occupational exposure to air pollution accounts for ~425,000 deaths worldwide (Balmes et al., 2003); in the USA, such diseases cost an estimated 7 billion dollars each year due to medical expenses and loss of productivity (Nelson et al., 2005; Schulte, 2005). Inhalable aerosol hazards, which contribute substantially to this burden, are present in virtually every industrial sector: construction (cement dust, fireproofing) (Ulvestad et al., 2000), manufacturing (oil mist) (Ameille et al., 1995; Greaves et al., 1997), machining of metal parts (Kreiss et al., 1996), polymer production, repair services, textile operations (National Institute for Occupational Safety and Health—NIOSH, 2008), agriculture and forestry (grain dusts, organic dusts in food animal production, flour dust, wood dust) (Omland, 2002; Spaan et al., 2006; Greskevitch et al., 2008), and mining and refining of minerals (especially metals) (NIOSH, 2008). Despite the link between aerosol hazards and respiratory disease, surveillance of occupational exposure remains challenging. Current methods for inhalable aerosol exposure assessment are limited by the costs of sampling and analysis, the complexity of certain sampler types, and issues of sampler representativeness and sample loss. Furthermore, concerns have been raised about the ability of inhalable samplers to match actual human aspiration efficiency in low-velocity environments (Kenny et al., 1999).
Aerosol inhalability is defined as the fraction of particles capable of penetrating into the head airways (and beyond) upon inhalation. The inhalable particulate mass (IPM) criterion was developed as an approximation of the aspiration efficiency of particles, as a function of aerodynamic diameter, for a human. The IPM was developed to define the desired sampling efficiency of inhalable aerosol samplers (Phalen et al., 1986; Sleeth and Vincent, 2012). The IOM, the Button, and the 37-mm closed-face cassette (CFC) are three commonly used aerosol samplers in the USA. However, only the IOM and Button have been shown to mimic the IPM over the range of particle sizes from 30 to 100 μm. The CFC, which is (incorrectly) referred to as a ‘total’ dust sampler, undersamples particles >30 μm in aerodynamic diameter (Kenny et al., 1999, Görner et al., 2010). In addition, the CFC and IOM sampler experience substantial particle deposition on their interior walls (Demange et al., 1990; Lafontaine et al., 1999). The US NIOSH discusses issues relating to the performance of different sampler designs in Chapter O of the Manual of Analytical Methods (Baron, 2003), but many OSHA methods call for use of a CFC sampler (OSHA, 2002).
The IOM was designed to achieve sampling efficiencies more consistent with the IPM criterion, and the Button sampler was found to closely approximate the IPM under the right operating conditions. However, in the USA, occupational hygienists have been slow to adopt these samplers, due in part to their cost, complexity, and other operational concerns. The Button sampler has a retail price of $249.00 and the IOM $85.00 (conductive plastic) or $269.00 (stainless steel), whereas the CFC costs ~$1.00. This cost differential has made the CFC attractive in the marketplace, especially because the sampler is considered disposable. For practical purposes, the IOM and Button are not disposable (due to their high retail price); they must be shipped back to the user following laboratory analysis and they may become contaminated following sampling of certain substances. For example, a device that has measured detectable levels of beryllium is often removed from service over concerns of contamination (Aerosol-Technology-Committee, 2011).
The IOM and Button samplers also have issues related to their complexity and ease-of-use. The IOM sampler is the most challenging to assemble and maintain. There are seven components to the IOM sampler necessary for it to be used correctly (Fig. 1), including three different O-rings. The Button sampler is simpler than the IOM; however, the filter must be removed immediately after sampling (to reduce losses during shipping and handling), which increases the risk of sample contamination in the field. Button samplers also have difficulty aspirating droplets or aerosols generated from wet methods (Koehler et al., 2012).
Figure 1.
Left: Disassembled IOM sampler. Right: Exploded view of the new sampling system (a) inlet cover, (b) inlet, (c) capsule and filter, and (d) housing.
A particularly innovative aspect of the IOM’s design was the incorporation of an internal sampling cassette, which retains aspirated particles that do not reach the filter or sampled particles that may become dislodged from the filter during use (such occurrences tend to increase with increasing particle size). As a result, however, the IOM experiences high rates of particle deposition on the walls of the internal cassette (Table 1). These wall deposits must be extracted or wiped from the inlet if chemical analysis is to be conducted. The IOM often has a relatively high limit of detection for gravimetric analysis (Paik and Vincent, 2002) due to static build-up (in the case of the plastic IOM) and because the internal cassette (which has substantially greater mass than the filter itself) must be weighed together with the filter to account for the fraction of particles depositing on the walls. Concerns have also been raised regarding the wide inlet of the IOM sampler.
Table 1.
Wall deposits in IOM samplers (as percent of total sample) from previous studies.
| IOM wall losses | Particle characteristics | Study |
|---|---|---|
| 25–44% | Four near-monodisperse aerosols evaluated ranging from ~6 to ~34 µm | (Mark, 1990) |
| Up to 25% | Nine near-monodisperse aerosols evaluated ranging from ~7 to ~100 µm | (Kenny et al., 1997) |
| 20–55% | Six near-monodisperse aerosols evaluated ranging from ~6.9 to ~76.0 µm | (Witschger et al., 2004) |
| 24–37% | Polydisperse | (Liden et al., 2000) |
| 1–33% | Polydisperse | (Hetland and Thomassen, 1993) |
Studies have reported that the IOM is sensitive to wind speed, especially for large particles (Li et al., 2000) and uncertainties remain about the representativeness of the current IPM curve in low velocities (<0.3 m s−1) (Lidén and Harper, 2006; Sleeth and Vincent, 2012). The IPM curve was developed following wind tunnel studies at relatively high free-stream velocities (>0.5 m s−1), whereas typical wind speeds in most indoor workplaces are on the order of 0.1–0.2 m s−1 (Baldwin and Maynard, 1998). Inhalability studies at low velocities report much higher aspiration curves compared to the IPM criterion, which has led to a proposed new low-velocity inhalability curve (Aitken et al., 1999). This curve is thought to be more representative of particles aspirated by manikins, and thus workers, at lower and more typical wind velocities (Aitken et al., 1999; Kenny et al, 1999; Sleeth and Vincent, 2012).
These limitations of current personal samplers highlight a need for a new generation of inhalable aerosol samplers that are inexpensive, simple, disposable, and compatible with the proposed low-velocity IPM sampling criterion; developing such a sampler has been the focus of this work.
METHODS
Sampler design
A new personal aerosol sampler was designed to meet the following goals: accurate assessment of workplace aerosols when compared to the proposed low-velocity IPM, simple to use, and disposable. This sampler employs a 37-mm filter (similar to the CFC) and is composed of four primary components (Fig. 1): inlet cover, inlet, capsule-style filter, and housing with mounting brackets. The inlet (Fig. 1b) is designed to aspirate aerosols with the same efficiency as the proposed low-velocity inhalability curve at a sampling flow rate of 2 l min−1.
The inlet is designed to seal through a ‘press-fit’, eliminating the need for threads or gaskets. Press-fit is a type of sealing that occurs when two surfaces are pressed together and held by material deformation and/or friction. An example of press-fit is the cork in the neck of a bottle; the CFC also seals by using press-fit.
The tested prototypes were made by stereo-lithography rapid prototyping (3D Systems, Rock Hill, SC, USA) using a conductive plastic (Acura 25). However, for mass production, the inlet and housing are designed for injection molding using conductive polyethylene or similar thermoplastic. The inlet cover (Fig. 1a) creates a seal over the inlet to prevent particle loss and contamination during transport/shipping. The inlet cover removes the need for handling the filter outside the laboratory.
A capsule-style filter (Fig. 1c) was designed to account for particle losses within the sampler itself (i.e. internal walls losses). The single use capsule consists of thin-film polycarbonate molded using a thermal vacuum-forming technique (Klein, 2009). The outer ring of the capsule contains a 2-mm flange that mates with the outer diameter of the sampling filter; the two components are designed to be weighed (or chemically analyzed) together. In this regard, the capsule may be sealed to the filter using the compressive force of the inlet or chemically bonded to the filter surface using toluene as a welding reagent. This configuration also minimizes cross-contamination as all parts are disposable.
The capsules can be bonded to numerous filter media (Fig. 1c), which allows them to be tailored for different analyses. Filter media that have been successfully bonded to the capsules include glass fiber, quartz, mixed cellulose ester, and polytetrafluoroethylene (PTFE/Teflon). Theoretically, a bonded capsule and filter could be disassembled, but this is impractical in most cases. The low cost and disposable nature of the capsules makes single use most appropriate. The capsule design can be applied to measurements beyond simple gravimetric analysis. For example, chemical and biological analyses can be conducted through typical solvent rinse/extraction techniques. As the applicability of rinse/extraction techniques depends on the composition of the sample, further development of such techniques for the new capsule is the subject of ongoing research and testing.
The housing (Fig. 1d) was also designed for injection molding using conductive thermoplastic. The base of the housing contains a series of raised ridges to distribute airflow evenly across the sampling filter. The housing contains a hose barb for connection to a personal sampling pump, oriented so the sampler lays flat against the worker’s torso, and external brackets for mounting within the worker’s breathing zone.
Sampling efficiency
Particle aspiration and sampling efficiencies of the new sampler were evaluated at the Rocky Mountain Center for Occupational & Environmental Health wind tunnel lab at the University of Utah in Salt Lake City, UT. Sampler efficiency was tested in a low-velocity wind tunnel equipped with a rotating manikin matching average human dimensions (Fig. 2). The manikin was neither breathing nor heated; however, previous studies conducted in the wind tunnel found only minor differences between tests conducted with a heated versus unheated manikin (Schmees et al., 2008a). The manikin was wearing a laboratory coat to give more representative airflow patterns and particle bounce. Schmees et al. (2008b) found aerosol concentrations at the manikin to be within 10% of each other; statistical differences were found only for the largest particles (>68 µm).
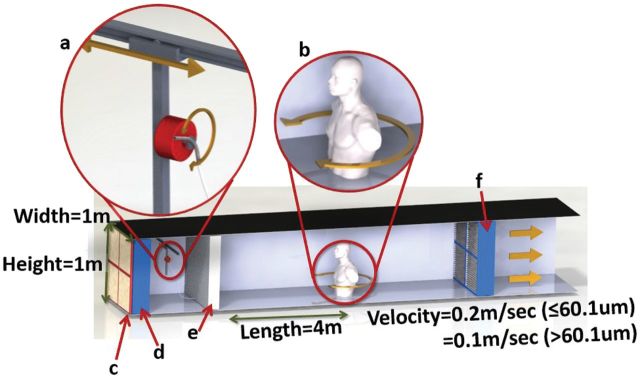
Figure 2.
Low-velocity wind tunnel used for testing the new sampler design. The tunnel was operated at 0.1 and 0.2 m s−1 (particle size dependent), with particles distributed using a TOPAS Solid Aerosol Generator 410 (TOPAS, Dresden, Germany). (a) Particles between 9.5 and 60.1 µm were distributed through a dispersion tube that transverse the width of the tunnel and oscillated through a 45° arc. Larger particles were dispersed from the top of the wind tunnel. (b) Samplers were attached to the lapel of a manikin that rotated clockwise 360° and then 360° counterclockwise. The manikin was 4 m from the flow conditioner. The tunnel includes (c) pre-filters, (d) high efficiency particulate air filters, (e) a flow conditioner, and (f) post-filters.
A wind speed of 0.2 m s−1 was used for particles with diameters between 9.5 and 60.1 µm, where particle injection upstream of the mannequin provided uniform distribution of particles near the manikin. Larger particles, 74 and 89.5 µm, needed to be tested at 0.1 m s−1 with upstream and overhead particle injection into the wind tunnel (Schmees et al., 2008b; Sleeth and Vincent, 2012). The lower wind tunnel velocity for tests of the larger particle sizes resulted in uniform distribution across the wind tunnel cross section, where at a wind speed of 0.2 m s−1, the test-to-test uniformity was poor.
As indoor wind speeds are typically >0.2 m s−1 (Baldwin and Maynard, 1998) we elected to not test the sampler at higher winds speeds as part of this proof-of-concept work. Testing of the sampler at higher wind speeds (which is appropriate for outdoor workplaces) as well as testing under calm-air settings will be the subject of future research. The wind tunnel was operated at near atmospheric pressure and temperature. Details of the wind tunnel, manikin, and concentration uniformity have been described elsewhere (Schmees et al., 2008b).
Samplers were affixed to the upper torso of the manikin and operated at 2 l min−1 by personal sampling pumps connected to the manikin’s back. Isokinetic samplers were operated at either 0.7 l min−1 (at a wind speed of 0.2 m s−1) or 0.55 l min−1 (at a wind speed of 0.1 m s−1); these samplers were used as our reference concentration when calculating the collection efficiency of the new sampler design. The isokinetic samplers were located at a distance 0.75 m upstream from the manikin. Sample pumps were calibrated using a BIOS DryCal flow meter (DC-Lite) before and after each test. Air speed in the wind tunnel was calibrated with a digital manometer (MA204E, Modus Instruments Inc, Clinton, MA, USA).
Test aerosols were generated using narrowly graded fused alumina powder (Duralum, Washington Mills, Niagara, NY, USA) at six particle sizes (Duralum F240, F280, F400, F600, F800, and F1200). Based on previous work, these were estimated to have aerodynamic diameters of 89.5 (F240 at 0.1 m s−1), 74, 60.1 (F240 at 0.2 m s−1), 44.3, 24.6, 12.8, and 9.5 µm, respectively, with nominal geometric standard deviations ranging from 1.19 to 1.38 (Schmees et al., 2008b). Schmees et al. (2008b) did not explicitly test F600; therefore an estimate of the aerodynamic diameter of F600 was interpolated from the data available.
Particles were dispersed using a TOPAS aerosol generator (Solid Aerosol Generator 410, Dresden, Germany) connected to an outlet tube. For particle sizes ranging from 9.5 to 60.1 µm the outlet tube traversed the width of the wind tunnel. The particle outlet tube oscillated across a 45° arc to ensure uniform vertical distribution. The dispersion tube covered the width of the tunnel every 15 s and completed a full oscillation every 45 s, ensuring uniform particle distribution in the tunnel. The 89.5 and 74 µm aerosols were dispersed from the top of the wind tunnel, using an injection system that also traversed the width of the wind tunnel to help distribute the particles along the horizontal plane.
Pallflex filters (Fiberfilm T60A20, Pall Corporation, Port Washington, NY, USA) were used for both the new sampler design and the isokinetic samplers. Two replicates were conducted at each particle size, with two isokinetic and three of the new samplers collecting samples simultaneously during each test. The isokinetic samplers were placed upwind of the manikin and all three test samplers were attached to the manikin torso. A total of 14 test replicates were conducted, and the order of tests were randomized.
The sampling efficiency of the new sampler was compared to data collected previously for the IOM sampler in similar wind tunnel experiments (Sleeth and Vincent, 2012). Sampler efficiency was calculated from the ratio of concentration measured by the sampler to the free-stream concentration as estimated from the isokinetic reference probe. Efficiency measurements at each size were based on the average of all instruments and test replicates using a pooled estimate of variance. The new sampler was also compared to the proposed low-velocity inhalability criterion in a similar manner. Statistical analyses were conducted in Matlab (R2011b, Mathworks). There was potential for substantial particle deposition in the inlets of the isokinetic samplers due to the large particle sizes and inlet velocities used. For particles between 9.5 and 60.1 µm, inlet deposition in the isokinetic samplers was measured using polydisperse ultraviolet fluorescent microspheres (UVYGPMS, Cospheric, Santa Barbara, CA, USA) of unit density and ranging in size from 10 to 100 μm. The fluorescent microspheres were dispersed using the same method as described for the Duralum dust. Microspheres depositing on both the filters and inlets of the isokinetic samplers were extracted with xylene into individual glass vials. Extraction efficiency was calculated by extracting filters and inlets with known masses of microspheres. Standard addition curves were used to determine the concentration of microspheres in each vial, which were then related to mass collected. A standard addition spike of 0.1mg ml−1 (microsphere mass in xylene) was used. Each standard curve consisted of four points of varying concentration analyzed in a 96-well plate using a FLX800 microplate reader (BioTek, Winooski, VT, USA) exciting at 360nm and reading at 530nm. For the largest particles (74 and 89.5 µm), a rinse method was used. Inlet tubes were rinsed with isopropyl alcohol into pre-weighed glass dishes. The isopropyl alcohol was left to evaporate for at least 12h and then the container was reweighed. The gravimetric based Duralam isokinetic measurements were scaled by the ratio of (filter + inlet)/filter to correct for inlet losses.
Wall deposits
As with the IOM sampler, particles deposited within the internal capsule of the new sampler are included in gravimetric mass measurements when the filter and capsule are weighed together. However, quantifying the extent of particle deposition to the walls is still important if chemical analyses of the filter capsule are desired. Thus, a separate set of tests was conducted to quantify particle deposition to the internal walls of the capsule. Wall deposits in the new sampler design were evaluated by count (as a function of particle size) and by total mass.
The Duralum dust concentrations collected by the samplers in Utah were extracted and analyzed using the same standard additional curve method discussed previously for the isokinetic sampler. These tests determined the total mass of material lost to the walls but did not determine where particles were deposited or if all particle sizes were deposited equally. The size distribution of particles depositing on the walls of the internal capsule and filter was evaluated in a separate set of tests using a low-velocity wind tunnel at Colorado State University, details of that experimental setup are described elsewhere (Koehler et al., 2011).
The wind speed was set at 0.2 m s−1 and samplers placed on a 51×32cm bluff body, with a blockage ratio of 16%. For these tests, the inside of the internal capsule was coated with a thin layer of silicone grease to retain deposited particles. Polydisperse fluorescent microspheres (UVYGPMS, Cospheric, Santa Barbara, CA, USA) were aspirated into the wind tunnel. Once the test dust had been dispersed in the wind tunnel, the pump to the new inhalable sampler was turned off, and the filter and internal capsule removed. The internal capsule was cut horizontally, then viewed and imaged using an epifluorescent microscope (95×; Orthoplan, Leica) and digital camera. ImageJ software (NIH, V1.46r) was used to analyze images, using a stage micrometer as reference. Sampling filters were imaged and analyzed using the same method. Projected particle diameters were calculated from the particle area values reported by ImageJ and particle size distributions were generated. Size distributions for particles depositing on the internal capsule were compared to size distributions for particles depositing on the filter.
Capsule-filter stability
Gravimetric stability of the capsule-filter assembly is important for sampler practicality and gravimetric limit of detection. Two aspects of filter stability were considered: time-to-equilibrium after the filter and capsule assembly is chemically bonded and measurement repeatability over time. The time-to-equilibrium affects how quickly a capsule and filter can be used following assembly. The mass of the capsule and filter assembly will decrease as the bonding agent (toluene) evaporates. Although equilibrium time is less important in the case of mass production, the simple assembly of the caps would allow individuals to bond the capsules themselves. The ability to bond capsules in-house opens up many possibilities with respect to filter type and filter handling that a study might require.
Six filter-capsule assemblies were chemically bonded then immediately weighed on a microbalance with 1 μg resolution (MX5, Mettler Toledo, Columbus, OH, USA). Three filter-capsule assemblies were stored at 20°C and three at 70°C (the latter to test heated drying). Each assembly was periodically reweighed until their masses stabilized. Following equilibrium, the filter capsules were weighed twice per day for a 5-day period to estimate measurement repeatability and associated gravimetric detection limits. The gravimetric limit of detection for the capsule and filter assemblies was designated as three times the standard deviation of these repeated measures.
RESULTS
Sampling efficiency
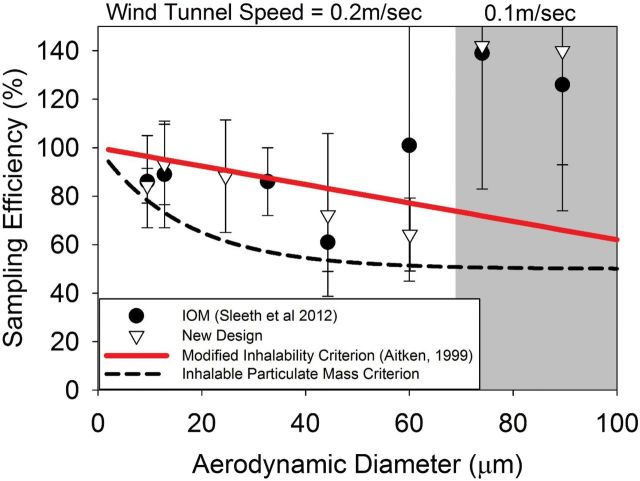
The sampling efficiency of the new inhalable sampler was found to be within ±13% of the proposed low-velocity inhalability criterion for the four smaller particle sizes tested (Fig. 3). Both the IOM and the new sampler substantially over sampled at the larger particle sizes (74 and 89.5 μm), which were only evaluated at the lower wind speed of 0.1 m s−1.
Figure 3.
Sampling efficiency of capsules compared to previous IOM studies and the modified inhalability criterion. Error bars represent pooled variance of all samplers and replicate tests at each particle size evaluated.
Wall deposition
A substantial percentage (60.2±11.2%) of the large particle sizes from the polydisperse microspheres were collected on the interior of the capsule compared to the filter itself (Fig. 4). Particle deposition on the walls of the internal capsule was uniformly distributed along the circumference of the internal cap walls. The majority of the particles deposited along the exterior inlet lip that protrudes outwards from the capsule (i.e. the leading edge of the inlet). The size distribution of particles deposited on the internal walls (count median diameter GM = 49 μm; geometric standard deviation (GSD) = 1.5) was similar to that on the filter (GM = 58 μm; GSD = 1.4). Size distributions along the interior of the internal cap were similar. The comparatively broader distribution (GSD = 1.5 versus GSD = 1.4) may be a result of multiple phenomena such as static charge and particles bouncing from the filter.
Figure 4.
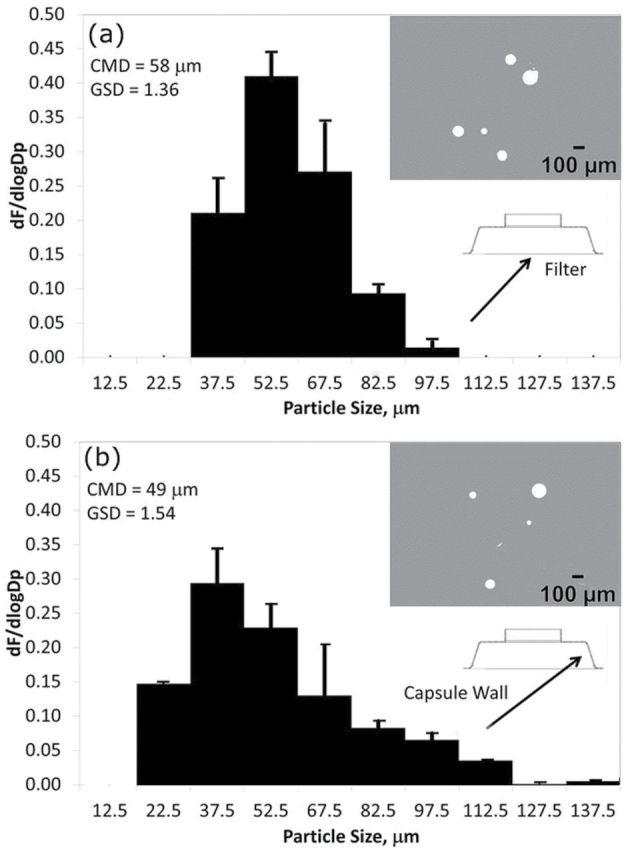
Size distribution of particles collected on the (a) filter and (b) interior capsule walls. The plots represent the size distribution of particles collected on the walls and filter. The y-axis is the log-transformed fraction of particles in each histogram bin.
Capsule stability and limit of detection
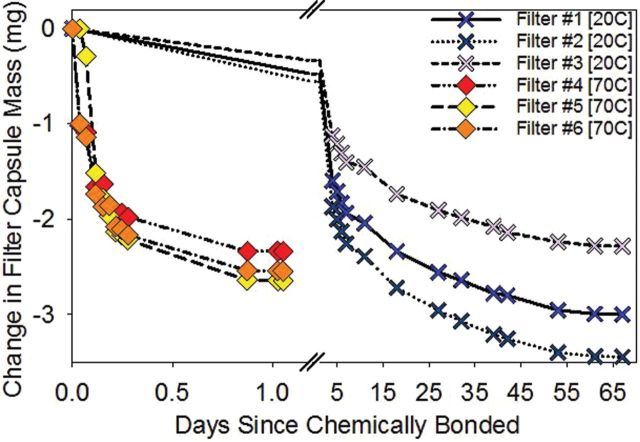
The capsule and filter assemblies conditioned at room temperature (20°C) reached equilibrium in ~65 days, with the majority of mass change occurring in the first week (Fig. 5). Capsules and filter assemblies conditioned at elevated temperature (70°C) required only 25h to reach equilibrium. The decrease in equilibration time at elevated temperature supports the hypothesis that toluene evaporation was the major cause of the mass drift. The limit of detection, i.e. the smallest collected mass that can be differentiated from the mass of the capsule and filter assembly after the toluene had fully evaporated, for the six filter-capsule assemblies tested ranged from 7 to 14 µg.
Figure 5.
Mass change of test capsule and filter assemblies after the chemical bonding process in days when stored at 20°C and when stored at 70°C.
DISCUSSION
Sampling efficiency
When the wind tunnel was operated at 0.2 m s−1, only the 9.5 μm particle size exhibited a significant difference in sampling efficiency from the proposed low-velocity inhalability criterion [one sample Student’s t-test; p(9.5 μm) = 0.007, p(12.8 μm) = 0.93, p(24.6 μm) = 0.77, p(44.3 μm) = 0.32, p(60.1 μm) = 0.60]. This difference, however, was not substantial (Fig. 3) and likely not meaningful in practice. However, the mean sampling efficiency at each particle size was below the proposed low-velocity inhalabiliy criterion, indicating that the new inhalable sampler could potentially underestimate exposure relative to the low-velocity criterion. The new inhalable sampler was slightly closer than the IOM to the proposed low-velocity inhalability criterion at 12.8, 44.3, and 60.1 μm; however, there were no statistical difference in sampling efficiencies between the two samplers (unpaired Student’s t-test; p(9.5 μm) = 0.84, p(12.8 μm) = 0.70, p(44.3 μm) = 0.34, p(60.1 μm) = 0.19) when sampling from 0.2 m s−1 air. These preliminary results indicate that the sampling efficiency of the new inhalable sampler is comparable to that of current inhalable samplers.
Although the oversampling of large particles (74 and 89.5 μm) could be an indication of a systematic problem with the IOM and the new sampler, the issue may also be due to the challenges associated with collecting large particles in low wind speeds. One possibility is the inherent difficulty with isokinetic sampling under these conditions, which would also explain why previous studies of the IOM and Button sampler in this wind tunnel produced similar results at such conditions (Sleeth and Vincent, 2012).
Therefore, it seems likely that the large increase in sampling efficiency is related to an artifact of the experimental procedure and not a flaw in the samplers themselves. In that case, it is most important to note that the new sampler produced similar results to the IOM under these conditions. The systematic oversampling of large particles does present a potential problem. Oversampling of particles overestimates the exposure workers are likely exposed to and could lead to groups implementing unnecessary and expensive exposure mitigation systems. Computer modeling is currently being conducted to explore the potential causes of this oversampling including wind speed effects, near-field flow patterns, and gravitational settling. This research is ongoing and is the subject of a forthcoming publication.
Wall deposition
Build-up of static charge on the capsules may have increased particle wall deposits, especially for small particles. Although static charge likely has little impact on the deposition of particles larger than 10 μm, this topic warrants further investigation. Several approaches for mitigating these effects are currently being investigated, including making the capsules out of conductive plastics amenable to vacuum forming and treating the capsules with static-dissipating surface coatings.
Capsule stability and limit of detection
Although the total mass change in each filter varied by over 1mg, all the filters in each set reached equilibrium in approximately the same amount of time. The difference in total mass change was due to each capsule and filter assembly absorbing a different quantity of toluene during assembly. If assembly was more controlled (as would occur in larger scale production) the amount of toluene used would be standardized, removing this variability.
Based on a limit of detection of <14 µg, the new sampler would be capable of evaluating workplace concentrations as low as 15 µg m−3 over an 8-h sample, which is substantially below most (gravimetric) occupational exposure limits.
A design decision was made to not make dissolvable capsules as the materials needed are often highly hydrophilic, making gravimetric measurements problematic. However, examples of dissolvable capsules can be found in the literature (Harper and Ashley, 2012). A similar approach could be applied to the capsule design for studies analyzing filters through chemical analysis.
The potential role of low-cost samplers in occupational exposure is complex. There are numerous components that affect the cost of exposure assessment that is unrelated to the upfront cost of the sampling equipment. However, sampler costs can also limit a study. The sampler that has been developed here is well suited to low-cost, high-volume production. Thus, this technology has the potential to increase the adoption (and throughput) of personal sampling in the workplace.
CONCLUSIONS
A new sampler was developed to measure personal exposure to inhalable particles and to mitigate many of the limitations of current sampler designs. The device is relatively simple to assemble and use, minimizes the effects of particle losses (by virtue of the internal capsule), and is both inexpensive and disposable (as needed). The intent of this design is to facilitate more widespread use of personal samplers capable of mimicking actual human aspiration. Such samplers may provide a more biologically relevant estimate of dose, as compared to designs like the CFC, which underestimate particle intake at larger sizes. An increase in the availability of personal exposure data will not only increase our understanding of the health risks associated with exposure but also improve our ability to mitigate worker exposure to particulate matter.
The new inhalable sampler closely matched the low-velocity inhalability criterion for particles ranging from 9.5 to 60.1 µm (and also matched the performance of the IOM sampler at all sizes). Therefore, the new inhalable sampler appears to be appropriate for evaluating personal exposure to inhalable aerosol hazards in the workplace within this range. Furthermore, the use of an internal capsule simplifies gravimetric analysis by eliminating the need to wipe or wash the internal sampler walls and inlets for particle deposits. The inlet cover may need to be wiped/washed in some instances (for example, transporting the sampler may result in particle release from the filter).
The new inhalable sampler is more practical to use compared to the IOM and Button sampler; it has substantially fewer components than the IOM and has been designed for low-cost, high-volume production. Future work is intended to quantify bypass leaking of the press-fit surfaces. The new sampler design improves upon the Button sampler by maintaining a simple design but incorporates an improved method of transporting samples. Incorporating the inlet cover in the design will minimize the amount of filter handling required in the field, thereby reducing the risk of sample contamination and loss.
The new design has the potential to retail at a cost much lower than that of currently available designs. There is the potential to create a paradigm shift in how personal exposure measurements are conducted by reducing the cost of samplers. With the potential of low production costs, the new sampler could be a viable option for small businesses, private citizens, and developing nations that have not historically monitored personal exposure to inhalable particulate matter.
FUNDING
This work was funded by a grant from the National Institute for Occupational Safety and Health (R01OH010295).
REFERENCES
- Aerosol-Technology-Committee. (2011) The state of the science of inhalable particles. A Science Symposium. American Industrial Hygiene Conference and Exposition. Portland, OR. [Google Scholar]
- Aitken RJ, Baldwin PEJ, Beaumont GC, et al. (1999) Aerosol inhalability in low air movement environments. J Aerosol Sci; 30: 613–26. [Google Scholar]
- Ameille J, Wild P, Choudat D, et al. (1995) Respiratory symptoms, ventilatory impairment, and bronchial reactivity in oil mist-exposed automobile workers. Am J Ind Med; 27: 247–56. [DOI] [PubMed] [Google Scholar]
- Baldwin PEJ, Maynard AD. (1998) A survey of wind speeds in indoor workplaces. Ann Occup Hyg; 42: 303–13. [DOI] [PubMed] [Google Scholar]
- Balmes J, Becklake M, Blanc P. (2003) Occupational contribution to the burden of airway disease (an official statement of the American Thoracic Society). Am J Respir Crit Care Med; 167: 787–97. [DOI] [PubMed] [Google Scholar]
- Baron P. (2003) Factors affecting aerosol sampling. NIOSH Manual of Analytical Methods. 4th ed. Cincinnati, OH: U.S. Department of Health and Human Services. [Google Scholar]
- Demange M, Gendre JC, Hervé-Bazin B, et al. (1990) Aerosol evaluation difficulties due to particle deposition on filter holder inner walls. Ann Occup Hyg; 34: 399–403. [Google Scholar]
- Görner P, Simon X, Wrobel R, et al. (2010) Laboratory study of selected personal inhalable aerosol samplers. Ann Occup Hyg; 54: 165–87. [DOI] [PubMed] [Google Scholar]
- Greaves IA, Eisen EA, Smith TJ, et al. (1997) Respiratory health of automobile workers exposed to metal-working fluid aerosols: respiratory symptoms. Am J Ind Med; 32: 450–9. [DOI] [PubMed] [Google Scholar]
- Greskevitch M, Kullman G, Bang KM, et al. (2008) Respiratory disease in agricultural workers: mortality and morbidity statistics. J Agromed; 12: 5–10. [DOI] [PubMed] [Google Scholar]
- Harper M, Ashley K. (2012) Preliminary studies on the use of acid-soluble cellulose acetate internal capsules for workplace metals sampling and analysis. J Occup Environ Hyg; 9: D125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetland S, Thomassen Y. (1993) Sampling and chemical characterization of aerosols in workplace air. Pure Appl Chem; 65: 2417–22. [Google Scholar]
- Kenny LC, Aitken RJ, Baldwin PEJ, et al. (1999) The sampling efficiency of personal inhalable aerosol samplers in low air movement environments. J Aerosol Sci; 30: 627–38. [Google Scholar]
- Kenny LC, Aitken R, Chalmers C, et al. (1997) A collaborative European study of personal inhalable aerosol sampler performance. Ann Occup Hyg; 41: 135–53. [DOI] [PubMed] [Google Scholar]
- Klein P. (2009) Chapter 4: The forming process. Fundamentals of plastics thermoforming. Synth Lect Mater Eng; 1: 29. [Google Scholar]
- Koehler KA, Anthony TR, van Dyke M, et al. (2011) A rotating bluff-body disc for reduced variability in wind tunnel aerosol studies. Ann Occup Hyg; 55: 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler KA, Anthony TR, Van Dyke M, et al. (2012) Solid versus liquid particle sampling efficiency of three personal aerosol samplers when facing the wind. Ann Occup Hyg; 56: 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss K, Mroz MM, Newman LS, et al. (1996) Machining risk of beryllium disease and sensitization with median exposures below 2 μg/m3 . Am J Ind Med; 30: 16–25. [DOI] [PubMed] [Google Scholar]
- Lafontaine M, Vu-Duc T, Delsaut P, et al. (1999) Aerosols deposits on the inner cassette walls during PAH sampling: underestimation of the inhaled fraction and of the occupational risk. Polycycl Aromat Comp; 17: 221–8. [Google Scholar]
- Li SN, Lundgren DA, Rovell-Rixx D. (2000) Evaluation of six inhalable aerosol samplers. AIHAJ; 61: 506–16. [DOI] [PubMed] [Google Scholar]
- Lidén G, Harper M. (2006) The need for an international sampling convention for inhalable dust in calm air. J Occup Environ Hyg; 3: D94–101. [DOI] [PubMed] [Google Scholar]
- Liden G, Melin B, Lidblom A, et al. (2000) Personal sampling in parallel with open-face filter cassettes and IOM samplers for inhalable dust-implications for occupational exposure limits. Appl Occup Environ Hyg; 15: 263–76. [DOI] [PubMed] [Google Scholar]
- Mark D. (1990) The use of dust-collecting cassettes in dust samplers. Ann Occup Hyg; 34: 281–91. [DOI] [PubMed] [Google Scholar]
- Nelson DI, Concha-Barrientos M, Driscoll T, et al. (2005) The global burden of selected occupational diseases and injury risks: methodology and summary. Am J Ind Med; 48: 400–18. [DOI] [PubMed] [Google Scholar]
- NIOSH. (2008). Work-related lung disease surveillance report, 2007. USA: National Institute for Occupational Safety and Health, Department of Health and Human Services. [Google Scholar]
- Omland O. (2002) Exposure and respiratory health in farming in temperate zones-a review of the literature. Ann Agric Environ Med; 9: 119–36. [PubMed] [Google Scholar]
- OSHA. (2002). Metal & metalloid particulates in workplace atmospheres (atomic absorption). OSHA Method ID-121. Occupational Safety & Health Administration. [Google Scholar]
- Paik S, Vincent JH. (2002) Filter and cassette mass instability in ascertaining the limit of detection of inhalable airborne particulates. AIHAJ; 63: 698–702. [DOI] [PubMed] [Google Scholar]
- Phalen RF, Hinds WC, John W, et al. (1986) Rationale and recommendations for particle size-selective sampling in the workplace. Appl Ind Hyg; 1: 3–14. [Google Scholar]
- Schmees DK, Wu Y-H, Vincent JH. (2008a) Visualization of the airflow around a life-sized, heated, breathing mannequin at ultralow windspeeds. Ann Occup Hyg; 52: 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmees DK, Wu Y-H, Vincent JH. (2008b) Experimental methods to determine inhalability and personal sampler performance for aerosols in ultra-low windspeed environments. J Environ Monit; 10: 1426–36. [DOI] [PubMed] [Google Scholar]
- Schulte PA. (2005) Characterizing the burden of occupational injury and disease. J Occup Environ Med; 47: 607–22. [DOI] [PubMed] [Google Scholar]
- Sleeth DK, Vincent JH. (2012) Performance study of personal inhalable aerosol samplers at ultra-low wind speeds. Ann Occup Hyg; 56: 207–20. [DOI] [PubMed] [Google Scholar]
- Spaan S, Wouters IM, Oosting I, et al. (2006) Exposure to inhalable dust and endotoxins in agricultural industries. J Environ Monit; 8: 63–72. [DOI] [PubMed] [Google Scholar]
- Ulvestad B, Bakke B, Melbostad E, et al. (2000) Increased risk of obstructive pulmonary disease in tunnel workers. Thorax; 55: 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschger O, Grinshpun SA, Fauvel S, et al. (2004) Performance of personal inhalable aerosol samplers in very slowly moving air when facing the aerosol source. Ann Occup Hyg; 48: 351–68. [DOI] [PubMed] [Google Scholar]