Soluble TNFR2 production during influenza infection, with emphasis on CD8+ T cells.
Keywords: adaptive immunity, cytokine regulation, host response, viruses
Abstract
Elevated levels of solTNFR2 are observed in a variety of human pathophysiological conditions but regulation of TNFR2 levels during disease is not well understood. We found that solTNFR2 levels were increased following influenza infection or live-attenuated influenza virus challenge in mice and humans, respectively. As influenza-specific CD8+ T cells up-regulated expression of TNFR2 after infection in mice, we hypothesized that CD8+ T cells contributed, in part, to solTNFR2 production after influenza infection and were interested in the mechanisms by which CD8+ T cells regulate TNFR2 shedding. Activation of these cells by TCR stimulation resulted in enhanced shedding of TNFR2 that required actin remodeling and lipid raft formation and was dependent on MAPK/ERK signaling. Furthermore, we identified ADAM17 as the protease responsible for TNFR2 shedding by CD8+ T cells, with ADAM17 and TNFR2 required in "cis" for shedding to occur. We observed similar activation thresholds for TNF-α expression and TNFR2 shedding, suggesting that solTNFR2 functioned, in part, to regulate solTNF-α levels. Production of solTNFR2 by activated CD8+ T cells reduced the availability of solTNF-α released by these cells, and TNFR2 blockade during influenza infection in mice enhanced the levels of solTNF-α, supporting this hypothesis. Taken together, this study identifies critical cellular mechanisms regulating TNFR2 shedding on CD8+ T cells and demonstrates that TNFR2 contributes, in part, to the regulation of TNF-α levels during infection.
Introduction
TNFR2 is a member of the TNFR superfamily, a family of proteins that functions to regulate T cell activation, proliferation, and survival [1]. TNFR2 mediates these effects by binding to mem- or solTNF-α [2–4]. TNFR2 is expressed on the surface following activation of naive CD8+ T cells, where it can function as a costimulatory molecule, lowering the threshold for activation and enhancing proliferation [5–9]. TNFR2−/− initially impairs T cell proliferation and survival, a result of, in part, reduced expression of IL-2 [9, 10]. This early defect in survival can be overcome with the addition of exogenous IL-2, and after becoming effector cells, TNFR2−/− CD8+ T cells are highly resistant to activation-induced cell death [7, 11]. These studies suggest that TNFR2 expression has an important role in CD8+ T cell activation and turnover.
One mechanism by which CD8+ T cells can regulate the effects of TNFR2 signaling is to decrease surface expression of TNFR2 in a process known as ectodomain shedding [12]. Activation of CD8+ T cells by TCR stimulation results in the production of solTNFR2 with a concomitant decrease in memTNFR2 expression [5, 6, 12]. The release of TNFR2 from the cell surface may reduce the sensitivity of the activated CD8+ T cell to the effects of TNF-α. Additionally, the released solTNFR2 is bioactive and can bind solTNF-α to regulate TNF-α signaling [13–15]. Regulation of solTNF-α is critically important in health and disease, as solTNF-α levels often determine the extent of injury during pathophysiological processes [16, 17]. Interestingly, increased levels of solTNFR2 have been observed in a variety of pathophysiological processes, including systemic lupus erythematosus, rheumatoid arthritis, and chronic viral hepatitis, suggesting that solTNFR2 regulation of solTNF-α bioavailability may impact disease progression and that the levels of solTNFR2 may reflect the extent of immune responses [18–20].
ADAM17 has been implicated in the shedding of TNFR2 from neutrophils, macrophages, and other cell types [21, 22]. Whereas it has been reported that CD8+ T cells express ADAM17 [23, 24], its role in shedding TNFR2 from activated CD8+ T cells has not been investigated. The regulation of ADAM17-mediated processing is complex and requires coordinated temporal and spatial signaling for shedding of TNFR2 to occur. These signals include phosphorylation of ADAM17, localization of ADAM17 and its substrates to lipid rafts, and dissociation from its endogenous inhibitor [25–29]. ADAM17 has been shown to mediate a constitutive level of shedding of its ligands, but different stimuli can up-regulate activity of ADAM17 and increase ligand shedding [30]. Notably, distinct stimuli elicit disparate signaling pathways that are responsible for the activation-dependent increase in ADAM17 activity [26, 31, 32]. A previous study investigating shedding of TNFR2 by human CD8+ T cells used the nonphysiologic stimulus PMA in combination with anti-CD3 to stimulate the cells [12]. In light of these interesting findings, an important objective of our study was to investigate how physiologic stimuli regulate shedding of TNFR2 by CD8+ T cells.
Our interest in understanding the regulation of ADAM17 and mechanisms of TNFR2 shedding by CD8+ T cells originates from our studies investigating the shedding of TNF-α by CD8+ T cells during influenza infection [16]. Herein, we report that ADAM17 is the principal protease required for TNFR2 shedding induced by physiologic TCR signaling in influenza-specific, effector CD8+ T cells. We also elucidate key signaling intermediates between TCR agonism and ADAM17-mediated TNFR2 shedding and discuss the importance of TNFR2 shedding in regulating the availability of solTNF-α during influenza infection.
MATERIALS AND METHODS
Reagents
TAPI was purchased from Peptides International (Louisville, KY, USA). Brefeldin A was purchased from LC Laboratories (Woburn, MA, USA). ERK1/2 selective inhibitor U0126 and antibodies for phospho-p44/p42 MAPK ERK1/2 (4370) and p44/p42 MAPK ERK 1/2 (9102) were from Cell Signaling Technologies (Danvers, MA, USA). Actin polymerization inhibitors latrunculin A and cytochalasin D were purchased from Enzo Life Sciences (Farmingdale, NY, USA). Lipid raft disrupters, methyl-β-cyclodextrin and filipin III, PMA, and FITC-conjugated CtxB subunit were purchased from Sigma-Aldrich (St. Louis, MO, USA). The following antibodies were from BioLegend (San Diego, CA, USA) as unconjugated or conjugated to FITC, PE, PerCP/Cy5.5, PE-Cy7, APC, APC-Cy7, or Pacific Blue: CD3 (17A2), CD8 (5.3-6.7), CD44 (IM7), CD62L (MEL-14), CD69 (H1.2F3), TNF-α (MP6-XT22), IFN-γ (XMG1.2), TNFR2 (TR75-89), donkey anti-rabbit (Poly4064), goat anti-Armenian hamster IgG (Poly4055), and β-actin (Poly6221). Goat anti-Armenian hamster IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Anti-mouse CD16/32 was from DartLab (Lebanon, NH, USA). APC-conjugated tetramer specific for the immunodominant, H-2Db-restricted NP366–374 epitope of A/PR/8/34 influenza A virus was prepared by the NIH Tetramer Core Facility (Atlanta, GA, USA).
Mice
C57BL/6 (WT) mice and TNFR2−/− or Rag1−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). TNF-α−/− mice were purchased from Taconic Biosciences (Germantown, NY, USA). ADAM17+/− mice, expressing a deletion in the zinc catalytic domain of ADAM17, were kindly provided by Dr. Carl Blobel (Weill Cornell Graduate School of Medical Sciences, New York, NY, USA). ADAM17−/− chimeric mice were generated as described previously [16]. In brief, fetal livers were collected from ADAM17−/− mice and prepared into a single-cell suspension that was adoptively transferred by tail-vein injection into sublethally irradiated Rag1−/− mice. After immune reconstitution, mice were infected with influenza virus. All animal studies were conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committee at Geisel School of Medicine at Dartmouth (Lebanon, NH, USA).
CD8+ T cell culture
NP366–374-specific CD8+ T cells were generated as described previously [17]. In brief, 3 wk after infection with influenza A/PR/8/34 virus, mice were euthanized, spleens were collected, and a single-cell suspension was prepared. Splenocytes were cultured with irradiated syngeneic splenocytes pulsed with NP366–374 peptide in fresh Iscove’s complete media, supplemented with IL-2. Cells were restimulated in vitro with NP366–374 peptide-pulsed, irradiated syngeneic splenocytes to obtain bulk cultures of NP366–374-specific CD8+ T cells.
In vitro TNFR2 shedding assays
NP366–374-specific CD8+ T cells were stimulated with NP366–374 peptide, PMA, or plate-bound anti-CD3. Following stimulation, cell-free supernatant was collected and assayed by ELISA for production of solTNFR2 (eBioscience, San Diego, CA, USA), solTNF-α (BioLegend, San Diego, CA, USA), and IFN-γ (BioLegend). Cells were harvested, and memTNFR2 expression was determined by flow cytometry. In experiments examining the effect of different inhibitors on TNFR2 shedding, cells were preincubated for 30 min before stimulation. For TNFR-blocking experiments, cells were treated with TNFR2-blocking antibody (TR75-54.7; BioLegend) or nonblocking antibody (TR75-89; BioLegend) for 20 min before stimulation. For solTNFR2 reconstitution experiments, recombinant mouse solTNFR2 (R&D Systems, Minneapolis, MN, USA) was added to the culture medium at the indicated concentrations.
In vivo TNFR2 shedding assays
TNFR2−/− mice were intranasally infected with influenza A/PR/8/34 virus. Twenty-four hours after infection, NP366–374-specific CD8+ T cells were adoptively transferred by tail-vein injection. At the appropriate times after transfer, mice were euthanized, serum and BALF were collected, and ELISA was performed to measure solTNFR2 levels. To assess the kinetics of memTNFR2 and solTNFR2 during influenza infection, WT mice were intranasally infected with influenza A/PR/8/34 virus and at the appropriate times, serum and BALF were harvested for analysis of solTNFR2 levels, and cells were recovered from the airways and MLN for analysis of memTNFR2 expression. For TNFR2-blocking experiments, WT mice received 500 μg anti-TNFR2 (TR75-54.7; BioXCell, West Lebanon, NH, USA) or isotype control antibody by intraperitoneal injection on days 4 and 6 postinfection and were euthanized on day 7 postinfection to examine solTNF-α levels. To inhibit TNFR2 shedding, WT mice received 5 mg/kg TAPI intratracheally and were euthanized 6 h later to examine solTNFR2 and memTNFR2 expression.
Immunocytochemistry
NP366–374-specific CD8+ T cells were stimulated with NP366–374 peptide on poly-d-lysine-coated, glass-bottom dishes (In vitro Scientific, Sunnyvale, CA, USA). Following stimulation, cells were washed briefly and fixed in 4% formaldehyde. Cells were then washed and permeabilized with 0.1% saponin/PBS. Following permeabilization, cells were blocked with 10% goat serum in 0.1% saponin/PBS for 1 h. Cells were then incubated with primary antibodies and isotype controls for 1 h. After washing, cells were then incubated with secondary antibodies for 1 h in the dark. Cells were washed thoroughly and mounted in ProLong Gold with DAPI (Life Technologies, Gaithersburg, MD, USA). Confocal microscopy was performed with a Zeiss LSM 510 confocal microscope with a 63× objective lens.
Western blotting
Cell lysates were prepared and samples loaded on a 4–20% polyacrylamide Precise Protein Gel (Thermo Scientific, Rockford, IL, USA), and SDS-PAGE was performed according to the manufacturer's instructions. Proteins were then transferred onto an Immobilon-P membrane (Millipore, Billerica, MA, USA), and the membrane was probed with the indicated antibodies. Proteins were visualized by use of ECL Western blotting substrate (Pierce, Rockford, IL, USA), and band intensities were determined by use of ImageJ software (NIH, Bethesda, MD, USA).
Flow cytometry
Cells were first blocked with anti-mouse CD16/32 and then stained with specific antibodies or isotype controls, as described previously [16]. For intracellular cytokine staining, cells were maintained in brefeldin A until fixation and then permeabilized and stained with anti-TNF-α and anti-IFN-γ. Flow cytometry was performed on a FACSCalibur cytometer (BD Biosciences, San Jose, CA) or MacsQuant Analyzer (Miltenyi Biotec, San Diego, CA), and data were analyzed by use of FlowJo software (Tree Star, Ashland, OR, USA).
Human LAIV challenge
Nasal-swab specimens were obtained before immunization and on days 2, 4, and 7 after an initial dose of LAIV, as described previously [33], and assessed for solTNFR2 by ELISA (R&D Systems). Informed assent/consent was obtained from all participants and their parents by use of protocols and forms approved by the Committee for the Protection of Human Subjects at Dartmouth College. The study was monitored by the Regulatory Compliance and Human Subjects Protection Branch of the National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health, and registered with ClinicalTrials.gov (NCT01246999).
Statistical analyses
Statistical analyses were performed with GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Two-tailed, unpaired t-test with 95% confidence interval or Wilcoxon signed rank test with 95% confidence interval was used to analyze differences between groups, whereas 1- or 2-way ANOVA with 95% confidence interval was used to analyze differences among 3 or more groups or among groups over time, respectively. Data are presented as the mean ± sd.
RESULTS
solTNFR2 production is increased in humans after LAIV challenge
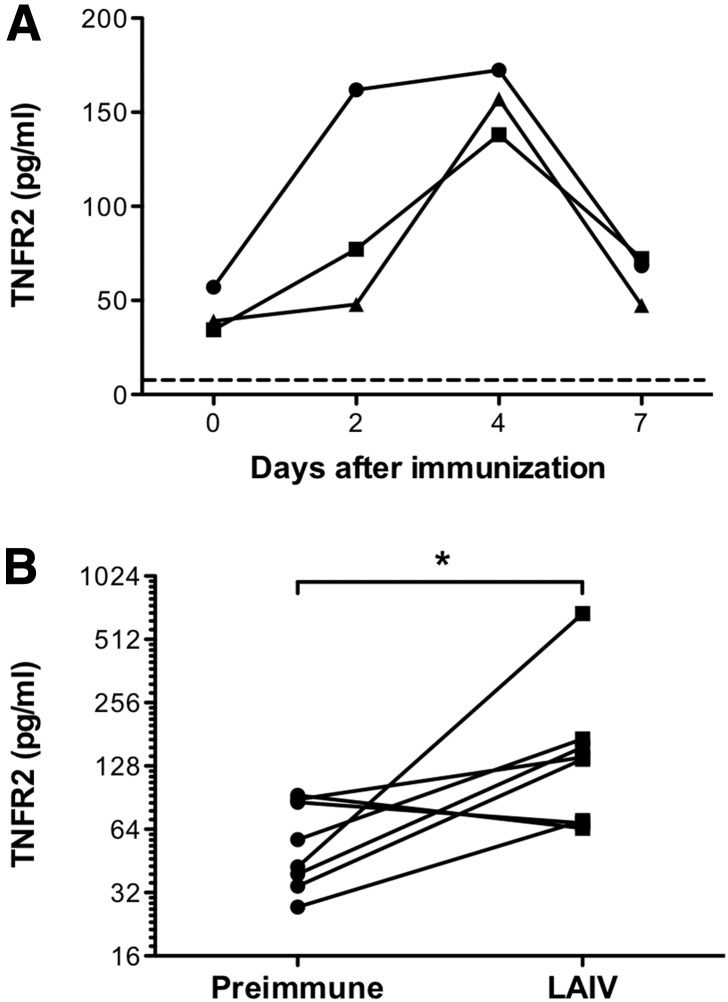
Expression of solTNFR2 is increased in a variety of inflammatory conditions in humans [18–20]; however, it was unknown whether influenza virus infection increased solTNFR2 levels. To test whether influenza virus increased solTNFR2 levels, we examined solTNFR2 levels from nasal-swab samples recovered from humans before and after challenge with LAIV. Before challenge, there were detectable levels of solTNFR2 in nasal-swab specimens (Fig. 1A and B). Following primary LAIV challenge, we observed an increase in solTNFR2 levels in the nasal-swab specimens recovered from a number of the patients (Fig. 1A), and when we examined the peak solTNFR2 levels that occurred after LAIV challenge, we found that there was a significant increase in solTNFR2 levels compared with preimmunization levels (Fig. 1B). Thus, similar to other inflammatory diseases and as proof of translational significance, solTNFR2 levels are increased after LAIV challenge in humans.
Figure 1. solTNFR2 levels are increased after LAIV challenge in humans.
Nasal-swab specimens were recovered from children before immunization or days 2, 4, and 7 after challenge with LAIV and assessed for solTNFR2 by ELISA. (A) Representative kinetics of solTNFR2 levels after LAIV challenge in 3 different patients. Dashed line represents the limit of detection for the ELISA (7.8 pg/ml). (B) Change in solTNFR2 levels from preimmune levels to the peak levels observed after LAIV challenge in 8 separate patients. *P < 0.05.
solTNFR2 production is increased after influenza infection in mice
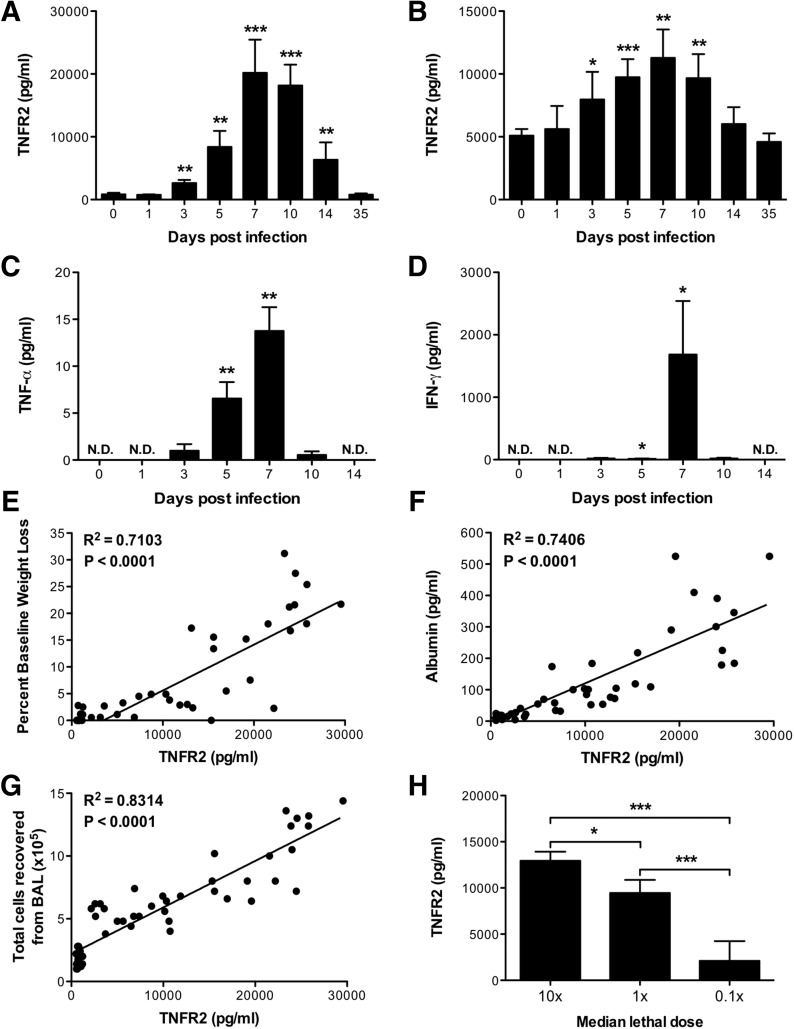
To investigate further the significance of increased solTNFR2 levels after influenza virus challenge, we used a murine model of experimental influenza infection, in which mice were inoculated with virus intranasally. Similar to humans, solTNFR2 was detectable in samples recovered from the airways and sera of mice before infection (Fig. 2 A and B). Following intranasal infection with influenza infection, we observed significant increases in airway and serum solTNFR2 levels (Fig. 2A and B). The peak levels of solTNFR2 occurred between days 7 and 10 postinfection, which also corresponded to a time at which we observed the peak airway levels of other proinflammatory cytokines, such as solTNF-α (Fig. 2C) and IFN-γ (Fig. 2D). Interestingly, there were significant, positive correlations between airway levels of solTNFR2 and weight-loss morbidity (Fig. 2E), airway albumin levels (Fig. 2F), a surrogate for vascular leakage, or total cells in the airway (Fig. 2G), suggesting that TNFR2 levels may be indicative of the extent of immune responses and severity of disease during influenza infection. To test the latter, we challenged mice with different doses of virus and found that increasing the initial dose of viral challenge raised airway levels of solTNFR2 (Fig. 2H). Thus, influenza infection in mice increases solTNFR2 expression, consistent with what we observed in humans after LAIV challenge, and solTNFR2 levels are a reflection of the extent of immune responses, which can be influenced by viral titers.
Figure 2. solTNFR2 levels are increased after influenza infection in mice.
WT mice were infected with A/PR/8/34 influenza virus, and solTNFR2 levels in the (A) airway and (B) serum were assessed by ELISA on the days indicated. Airway levels of (C) solTNF-α and (D) IFN-γ were analyzed by ELISA on the days indicated. The levels in influenza-infected mice were compared with baseline values from naive mice for statistical analysis (*P < 0.05; **P < 0.01; ***P < 0.005). N.D., not detected. The correlations of (E) percent baseline weight change, (F) airway albumin levels, or (G) total cells recovered from the airway with airway solTNFR2 levels throughout the course of infection were determined by linear regression analysis. The slope of each linear regression was found to be significantly nonzero (<0.0001), as calculated from an F test. (H) WT mice were infected with 10, 1, or 0.1 times the median lethal dose of A/PR/8/34 influenza virus, and on day 7 postinfection, solTNFR2 levels in the airway were assessed by ELISA. Data represent means ± sd. Data are representative of 2 independent experiments with 3–5 mice/group. *P < 0.05; ***P < 0.005.
TNFR2 is expressed on and shed by influenza-specific effector CD8+ T cells
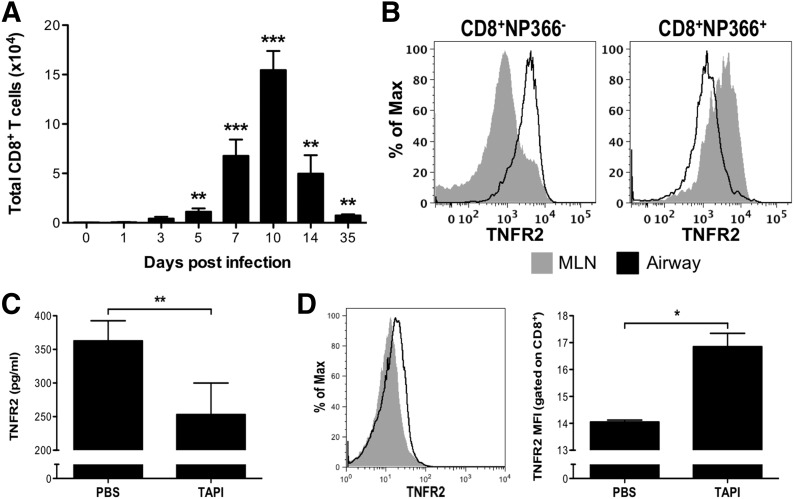
As it has been reported that memTNFR2 is expressed on influenza-specific CD8+ T cells in the lungs of infected mice [4, 34], we examined whether CD8+ T cells contributed to the increase in solTNFR2 levels following influenza infection in mice. CD8+ T cells were detectable in the airways as early as day 5 postinfection and peaked in absolute numbers between days 7 and 10 (Fig. 3A), which corresponded to the peak in airway and serum solTNFR2 levels (Fig. 2A and B). memTNFR2 was detectable on CD8+ T cells in the airways and lung-draining MLN, the site of CD8+ T cell priming, on day 7 postinfection (Fig. 3B). We found that the vast majority of CD8+ T cells expressing memTNFR2 in the MLN was NP366–374 specific, indicating that influenza-specific CD8+ T cells expressed memTNFR2 upon activation in the MLN before trafficking to the lung (Fig. 3B). As shedding of TNFR2 by human CD8+ T cells has been shown to be sensitive to metalloproteinase inhibition [12], we tested whether CD8+ T cells contributed to solTNFR2 expression in the influenza-infected lung by intratracheally administering a broad-spectrum metalloproteinase inhibitor, TAPI, on day 10 postinfection. TAPI treatment significantly reduced solTNFR2 levels in the airways (Fig. 3C), with a concomitant increase in surface expression of memTNFR2 on CD8+ T cells recovered from the airways (Fig. 3D), indicating that CD8+ T cells contributed to the increased levels of solTNFR2 after influenza infection. Consistent with previous reports [21, 22], TAPI treatment also increased surface expression of memTNFR2 on other immune cells (Supplemental Fig. 1), indicating that CD8+ T cells contributed, in part, to the increase in solTNFR2 levels in the influenza-infected lung.
Figure 3. CD8+ T cells express and shed TNFR2 during influenza infection.
(A) WT mice were infected with influenza virus, and on the days indicated, cells were recovered from the airways, and the absolute numbers of CD8+ T cells were determined by flow cytometry. The levels in influenza-infected mice were compared with baseline values from naive mice for statistical analysis (**P < 0.01; ***P < 0.005). (B) Cells were recovered from the MLN (gray) and airways (black line) on day 7 postinfection, and the expression of memTNFR2 on NP366–374-specific CD8+ T cells and all other CD8+ T cells was determined by flow cytometry. Alternatively, on day 10 postinfection, WT mice received TAPI or vehicle control intratracheally, and BAL was performed 6 h later. (C) Airway levels of solTNFR2 were assessed by ELISA. (D) memTNFR2 expression on airway CD8+ T cells was determined by flow cytometry, with a representative histogram (left) and quantified graph (right). MFI, Mean fluorescence intensity. Data represent means ± sd. Data are representative of 3 independent experiments with 3–5 mice/group. *P < 0.05; **P < 0.01.
ADAM17 is required for shedding of TNFR2 by effector CD8+ T cells
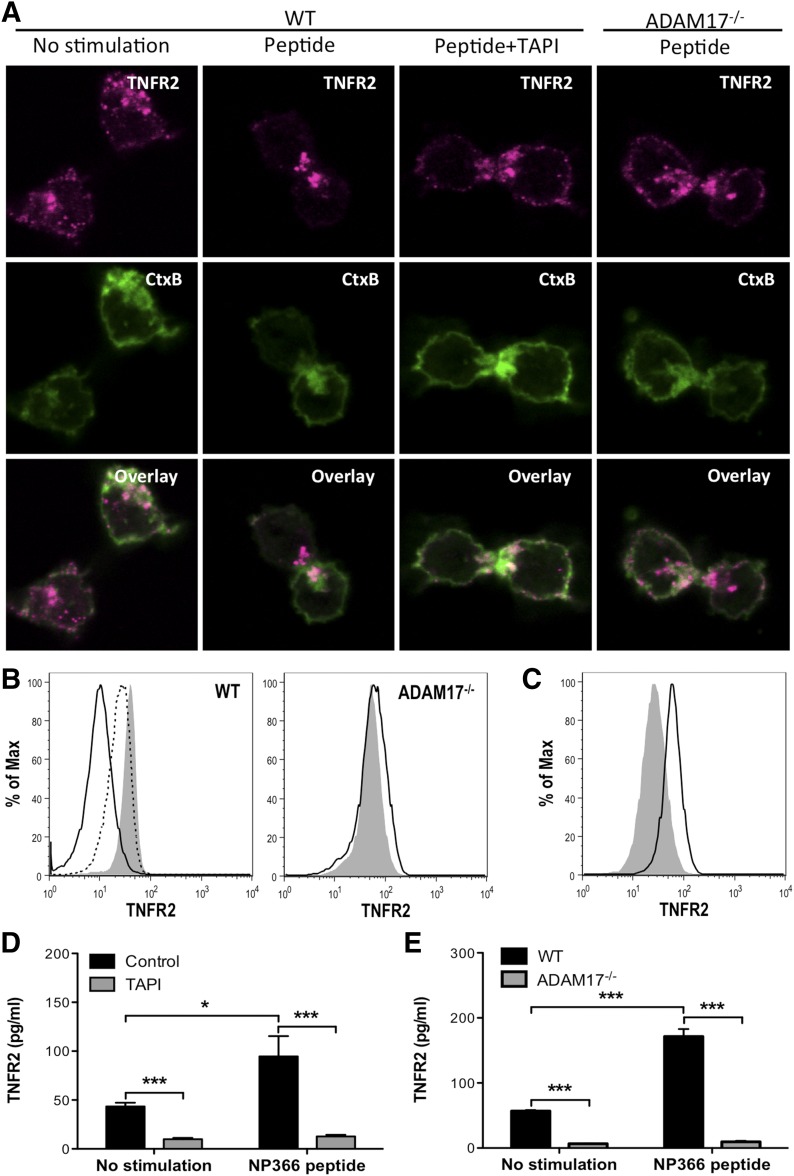
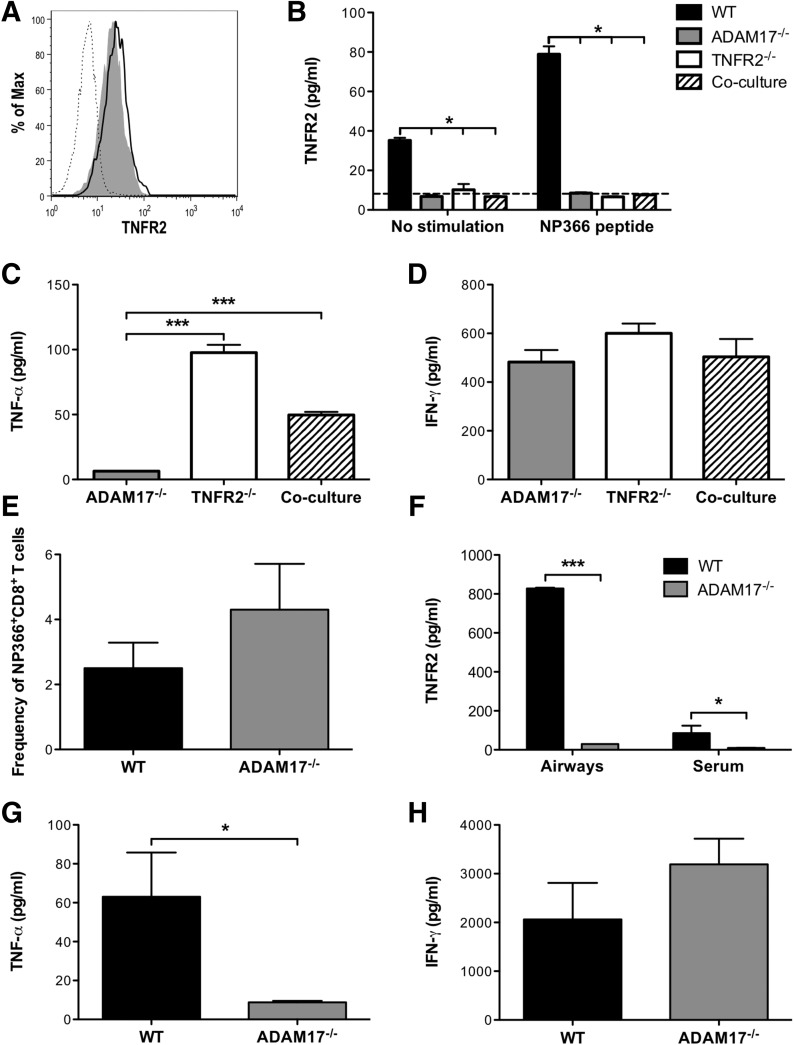
To examine further the regulation of TNFR2 shedding by influenza-specific CD8+ T cells, we generated bulk cultures of NP366–374-specific CD8+ T cells and examined their capacity to shed TNFR2 in vitro. TCR stimulation reduced memTNFR2 expression, which was blocked by TAPI treatment (Fig. 4A and B). Interestingly, TAPI treatment of unstimulated CD8+ T cells increased memTNFR2 expression (Fig. 4C), and treatment of CD8+ T cells with brefeldin A, which blocks protein trafficking, reduced memTNFR2 levels (Supplemental Fig. 2), indicating that TNFR2 was constitutively recruited to the surface and shed from effector CD8+ T cells. Furthermore, TAPI treatment inhibited constitutive and TCR-stimulated production of solTNFR2 (Fig. 4D). Whereas ADAM17 has been identified as a protease responsible for shedding TNFR2 on many cell types [21, 22], it was unknown whether it was responsible for shedding TNFR2 by CD8+ T cells. To test whether ADAM17 was required for TNFR2 shedding, we generated ADAM17−/− NP366–374-specific CD8+ T cells that expressed a deletion in the zinc catalytic domain of ADAM17, resulting in functional inactivation of ADAM17. Following TCR stimulation of ADAM17−/− CD8+ T cells, there was no decrease in memTNFR2 levels, in contrast to the decreased memTNFR2 levels observed on activated WT CD8+ T cells (Fig. 4A and B). Furthermore, constitutive and activation-induced solTNFR2 production was inhibited in ADAM17−/− CD8+ T cells compared with WT CD8+ T cells (Fig. 4E). Taken together, these data identify ADAM17 as the protease required for TNFR2 shedding by effector CD8+ T cells.
Figure 4. ADAM17 is required for TNFR2 shedding by influenza-specific CD8+ T cells.
TNFR2 shedding was examined in vitro on NP366–374-specific CD8+ T cells with NP366–374 peptide stimulation. (A) TNFR2 expression and CtxB binding were examined on unstimulated or peptide-stimulated and TAPI-treated, peptide-stimulated WT CD8+ T cells after 15 min of stimulation by confocal microscopy. Alternatively, TNFR2 and CtxB expression was examined on ADAM17−/− CD8+ T cells after 15 min of stimulation. (B) Representative histograms of memTNFR2 expression on unstimulated (gray), peptide-stimulated (black line), or TAPI-treated, peptide-stimulated (dashed line) WT or ADAM17−/− CD8+ T cells after 15 min of stimulation. (C) Unstimulated WT CD8+ T cells were left alone (gray) or treated with TAPI, and memTNFR2 expression was assessed 4 h later by flow cytometry. (D) Effects of TAPI on constitutive and peptide-stimulated solTNFR2 production by WT CD8+ T cells after 1 h of stimulation, as measured by ELISA. (E) Constitutive and peptide-stimulated solTNFR2 production by WT or ADAM17−/− CD8+ T cells after 1 h of stimulation, as measured by ELISA. Data represent means ± sd. Data are representative of 3 independent experiments with each condition conducted in triplicate. *P < 0.05; ***P < 0.005.
ADAM17 is required in cis for shedding of TNFR2 by CD8+ T cells
We next examined whether ADAM17 could shed TNFR2 in "trans" or "cis" in vitro by coculturing ADAM17−/− CD8+ T cells, which lack the functional enzyme but express the substrate on the surface with TNFR2−/− CD8+ T cells, which possess the functional enzyme but lack the substrate. We did not observe any decreases in memTNFR2 expression after TCR stimulation on ADAM17−/− CD8+ T cells alone or cocultured with TNFR2−/− CD8+ T cells (Fig. 5A). Coculture of ADAM17−/− CD8+ T cells with TNFR2−/− CD8+ T cells also resulted in no detectable production of solTNFR2 (Fig. 5B). Importantly, we observed solTNF-α production following TCR stimulation by TNFR2−/− CD8+ T cells alone and in the coculture conditions, indicating that ADAM17 was functional (Fig. 5C). Furthermore, IFN-γ production was not impaired in any of the conditions, suggesting that all of the CD8+ T cells were functionally active under these conditions (Fig. 5D). To test this in vivo, we adoptively transferred WT or ADAM17−/− NP366–374-specific CD8+ T cells into influenza-infected TNFR2−/− mice and examined solTNFR2 levels. We recovered a similar frequency and total number of transferred cells from the airways (Fig. 5E), indicating that WT and ADAM17−/− CD8+ T cells were capable of trafficking to the infected lung to recognize antigen. solTNFR2 was detectable in the airways and serum of TNFR2−/− recipients of WT CD8+ T cells (Fig. 5F). In contrast, TNFR2−/− recipients of ADAM17−/− CD8+ T cells had no detectable solTNFR2 levels (Fig. 5F), indicating that host ADAM17 was unable to cleave TNFR2 in trans on ADAM17−/− CD8+ T cells. Airway levels of solTNF-α were also markedly reduced in TNFR2−/− recipients of ADAM17−/− CD8+ T cells compared with WT CD8+ T cells (Fig. 5G). Importantly, no differences in the airway levels of IFN-γ were observed (Fig. 5H), suggesting that WT and ADAM17−/− CD8+ T cells were capable of being activated to mediate effector functions. Taken together, these observations reveal that ADAM17 and TNFR2 are required in cis on CD8+ T cells in vitro and in vivo for ADAM17 to mediate shedding of TNFR2.
Figure 5. ADAM17 and TNFR2 are required in cis for TNFR2 shedding by influenza-specific CD8+ T cells.
To test whether ADAM17 shed TNFR2 in cis or trans in vitro, ADAM17−/− NP366–374-specific CD8+ T cells were cocultured with TNFR2−/− NP366–374-specific CD8+ T cells with NP366–374 peptide stimulation for 4 h. (A) Representative histogram of memTNFR2 expression on unstimulated (gray) or peptide-stimulated (black line) ADAM17−/− NP366–374-specific CD8+ T cells during coculture conditions. Before coculture, TNFR2−/− CD8+ T cells were labeled with CFSE to distinguish between ADAM17−/− and TNFR2−/− CD8+ T cells, and memTNFR2 expression on TNFR2−/− CD8+ T cells (dashed line) is presented as a staining control. (B) ELISA was used to measure constitutive and peptide-stimulated solTNFR2 production by ADAM17−/− and TNFR2−/− CD8+ T cells, alone or in coculture conditions. The dashed line represents the limit of detection of the ELISA. WT CD8+ T cells were used as a positive control for TNFR2 shedding. ELISA was used to measure peptide-stimulated (C) solTNF-α and (D) IFN-γ production by ADAM17−/− and TNFR2−/− CD8+ T cells, alone or in coculture conditions. To test whether ADAM17 shed TNFR2 in cis or trans in vivo, influenza-infected TNFR2−/− mice received NP366–374-specific WT or ADAM17−/− CD8+ T cells, and BAL was performed on day 5 postinfection. (E) The frequency of transferred NP366–374-specific WT or ADAM17−/− CD8+ T cells was determined by flow cytometry. ELISA was used to measure (F) serum and airway levels of solTNFR2 and airway levels of (G) solTNF-α and (H) IFN-γ. Data represent means ± sd. Data are representative of 2 independent experiments with each condition conducted in triplicate or each group containing 3–4 mice. *P < 0.05; ***P < 0.005.
Shedding of TNFR2 is dependent on strength of TCR signaling and MAPK ERK1/2 activation
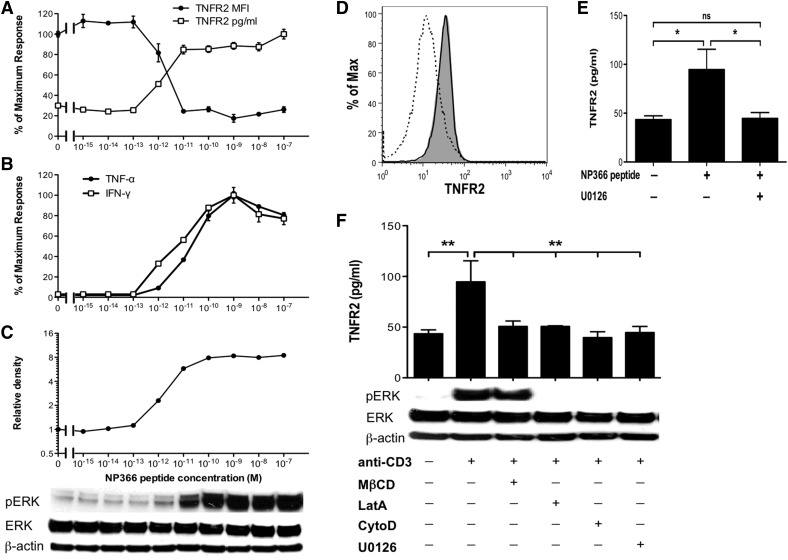
As ADAM17 is required for TNFR2 and TNF-α shedding by CD8+ T cells and the strength of TCR signaling can regulate the induction of different CD8+ T cell effector functions [35], we next examined the key signaling intermediates for TNFR2 shedding and cytokine production. With the use of increasing concentrations of NP366–374 peptide as a surrogate for increasing TCR signaling strength, we found that the activation threshold for TNFR2 shedding by NP366–374-specific CD8+ T cells occurred near a concentration of 10−12 M NP366–374 peptide (Fig. 6A). We observed a similar activation threshold for IFN-γ and TNF-α expression (Fig. 6B). As ADAM17-mediated processing of CD62L by CD8+ T cells was shown to be dependent on TCR signal transduction through the MAPK ERK1/2 pathway [32], and ERK1/2 signaling has also been shown to be important for trafficking, maturation, and activation of ADAM17 [25, 26], we next examined whether ERK1/2 signaling was important for TNFR2 shedding by effector CD8+ T cells. First, we examined the activation threshold for activation of ERK1/2 signaling and found that the threshold for ERK1/2 phosphorylation was similar to the activation threshold for TNFR2 shedding (Fig. 6C), indicating that ERK1/2 signaling and TNFR2 shedding were tightly regulated by TCR signaling. Next, we blocked TCR-stimulated ERK1/2 signaling with U0126, a highly selective inhibitor of ERK1/2, and found that U0126 treatment blocked the TCR-stimulated decrease in memTNFR2 levels (Fig. 6D) and TCR-stimulated solTNFR2 production (Fig. 6E). Treatment of cells with actin-remodeling inhibitors or cholesterol-depleting or -inhibitory agents, the latter that block lipid raft formation, also impaired ERK1/2 signaling and TNFR2 shedding (Fig. 6F), indicating that MAPK ERK1/2 signaling was critical for activation-induced TNFR2 shedding.
Figure 6. Strength of TCR signaling regulates ERK1/2-dependent TNFR2 shedding by influenza-specific CD8+ T cells.
NP366–374-specific WT CD8+ T cells were stimulated with increasing concentrations of peptide as a surrogate of increasing TCR signaling strength. (A) memTNFR2 expression and solTNFR2 production were examined after 1 h of peptide stimulation by flow cytometry and ELISA, respectively. (B) ELISA was used to measure solTNF-α and IFN-γ production after 4 h of peptide stimulation. (C) ERK1/2 phosphorylation (pERK) after 30 min of peptide stimulation was examined by Western blot with ImageJ analysis to determine relative band density. (D) Representative histogram of memTNFR2 expression on unstimulated (gray), peptide-stimulated (dashed line), or U0126-treated, peptide-stimulated (black line) WT CD8+ T cells after 1 h of peptide stimulation. (E) Effects of U0126 treatment on peptide-stimulated solTNFR2 production by WT CD8+ T cells after 1 h of stimulation, as measured by ELISA. ns, Not significant. (F) Effects of actin remodeling [latrunculin A (LatA) and cytochalasin D (CytoD)] or lipid raft [methyl-β-cyclodextrin (MβCD)] inhibitors on ERK1/2 phosphorylation and solTNFR2 production by WT CD8+ T cells after 1 h of stimulation. Data represent means ± sd. Data are representative of 2 independent experiments with each condition conducted in triplicate. *P < 0.05; **P < 0.01.
TNFR2 shedding by activated CD8+ T cells contributes to the regulation of solTNF-α availability
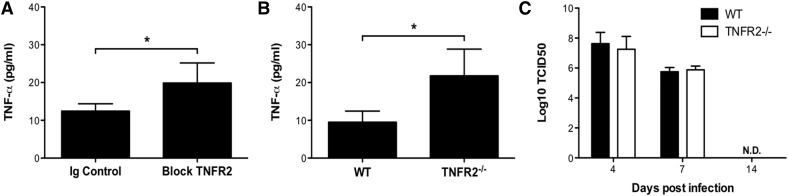
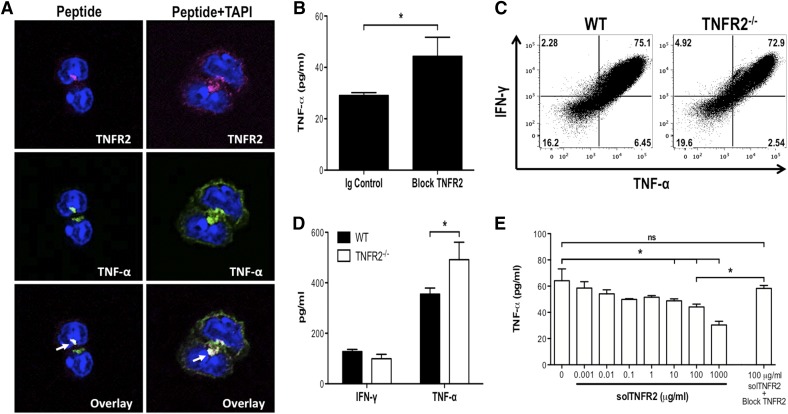
As we observed similar activation thresholds for TNF-α expression and TNFR2 shedding, we assessed the possibility that the increased solTNFR2 levels functioned, in part, to limit the availability of solTNF-α by use of antibody blockade of TNFR2 or mice lacking TNFR2. Antibody blockade or genetic TNFR2−/− resulted in increased levels of solTNF-α after influenza infection (Fig. 7A and B), suggesting that TNFR2 functioned, in part, to regulate solTNF-α levels. TNFR2−/− had no impact on the ability of mice to control or clear virus, indicating that the increased solTNF-α levels were not a result of increased viral titers (Fig. 7C). Next, we investigated whether TNFR2 shedding by activated CD8+ T cells could modulate the levels of CD8+ T cell-produced solTNF-α. TNFR2 and TNF-α were recruited to the immunologic synapse of activated CD8+ T cells (Fig. 8A), indicating that solTNFR2 would be spatially available to regulate solTNF-α levels. Moreover, TNFR2 shedding preceded TNF-α expression and release by activated CD8+ T cells (Supplemental Fig. 3A), consistent with previous reports [12]. Interestingly, memTNFR2 levels decreased over time, even in the presence of TAPI, and this may have been a result of, in part, an autocrine interaction between memTNFR2 and memTNF-α, as antibody blockade of TNFR2 (Supplemental Fig. 3B) or genetic deletion of TNF-α (Supplemental Fig. 3C) restored memTNFR2 levels in the presence of TAPI. These data demonstrate that TNFR2 shedding and TNF-α expression are tightly linked and suggest that loss of memTNFR2 from the activated CD8+ T cell may desensitize the cell to the effects of TNF-α, and the solTNFR2 that is released may affect the availability CD8+ T cell-produced TNF-α. To test the latter possibility, we treated activated WT CD8+ T cells with blocking antibody for TNFR2 and found that this enhanced the total levels of solTNF-α recovered in the supernatant (Fig. 8B). Next, we examined solTNF-α production in the complete absence of TNFR2. Whereas TNFR2−/− and WT CD8+ T cells produced similar total levels of intracellular TNF-α (Fig. 8C), we detected increased levels of solTNF-α in the supernatant of activated TNFR2−/− CD8+ T cells compared with WT CD8+ T cells (Fig. 8D). Addition of recombinant solTNFR2 to activated TNFR2−/− CD8+ T cells significantly reduced the level of solTNF-α detected in a dose-dependent manner, and this effect was abrogated with the addition of blocking antibody for TNFR2 (Fig. 8E). Taken together, these data suggest that shed TNFR2 from activated CD8+ T cells functions, in part, to regulate the availability of solTNF-α released from these activated cells.
Figure 7. TNFR2 contributes to the regulation of solTNF-α levels during influenza infection in mice.
(A) Influenza-infected WT mice received Ig control or anti-TNFR2 antibodies intraperitoneally on days 4 and 6 postinfection, and airway levels of solTNF-α were measured on day 7 postinfection by ELISA. (B) WT and TNFR2−/− mice were infected with influenza virus, and solTNF-α levels in the airways were measured on day 7 postinfection by ELISA. (C) Viral titers from WT or TNFR2−/− mice were determined from whole lung homogenates by use of the 50% tissue culture-infective dose (TCID50) method. Data represent means ± sd. Data are representative of 2 independent experiments with 3–5 mice/group. *P < 0.05.
Figure 8. Influenza-specific CD8+ T-cell produced solTNFR2 regulates the availability of solTNF-α.
To investigate the regulation of solTNF-α levels by solTNFR2 in activated CD8+ T cells, NP366–374-specific CD8+ T cells were stimulated in vitro with peptide (A) TNFR2 and TNF-α expression on peptide-stimulated or TAPI-treated, peptide-stimulated WT CD8+ T cells after 1 h of stimulation was examined by confocal microscopy. Arrows indicate colocalization of TNFR2 and TNF-α. (B) Effects of blocking antibody for TNFR2 on solTNF-α expression after 1 h of peptide stimulation of WT CD8+ T cells, as measured by ELISA. (C) Total intracellular production of TNF-α and IFN-γ by peptide-stimulated WT or TNFR2−/− CD8+ T cells after 4 h of stimulation, as assessed by flow cytometry. (D) ELISA was used to measure solTNF-α and IFN-γ production by peptide-stimulated WT or TNFR2−/− CD8+ T cells after 4 h of stimulation. (E) ELISA was used to measure solTNF-α levels after the addition of increasing concentrations of recombinant solTNFR2 to cultures of peptide-stimulated TNFR2−/− CD8+ T cells. Data represent means ± sd. Data are representative of 2 independent experiments, with each condition conducted in triplicate. *P < 0.05.
DISCUSSION
In this study, we demonstrated that effector CD8+ T cells constitutively express and shed TNFR2, and we identified ADAM17 as the protease responsible for shedding of TNFR2. Moreover, ADAM17 and TNFR2 were required in cis for shedding to occur. TCR-stimulated shedding required actin remodeling and lipid raft formation and was dependent on the strength of TCR signaling and the MAPK/ERK pathway. TNFR2 shedding was increased after influenza infection in mice, where it had a role in regulating the availability of solTNF-α. Additionally, we observed an increase in solTNFR2 levels after LAIV challenge in humans, suggesting that the shed receptor may have a role in regulating solTNF-α levels during influenza infection in humans.
TNFR2 expression was inducible and observed on recently activated influenza-specific CD8+ T cells in the lung-draining lymph node during influenza infection. TCR stimulation of these cells resulted in shedding of TNFR2 from the cell surface and increased levels of solTNFR2, consistent with previous studies that used human T cells [5, 6, 12]. TNFR2 was constitutively recruited to the surface of effector CD8+ T cells as inhibition of protein trafficking by brefeldin A reduced memTNFR2 levels and subsequently reduced activation-induced solTNFR2 production. This is similar to the reduced memTNFR1 and activation-induced solTNFR1 production observed in human endothelial cells treated with brefeldin A [36]. This demonstrates that effector CD8+ T cells actively maintain TNFR2 on the cell surface and may sensitize the cell to the effects of TNF-α. Thus, it has been hypothesized that TNFR2 shedding functions, in part, to desensitize an activated cell from TNFR2-dependent TNF-α signaling effects [37]. Several of our observations support this hypothesis. First, following activation, CD8+ T cells continuously shed TNFR2 and maintained low expression of the receptor on the cell surface, despite continuous trafficking of TNFR2 from intracellular stores to the cell membrane. Moreover, TNFR2 shedding preceded TNF-α expression and release. Second, contrary to a prior report that suggested myeloid-derived suppressor cell-expressed ADAM17 operated in trans to cleave CD62L on T cells [24], we found that ADAM17 functioned in cis to shed TNFR2, which would allow for stricter control of ligand and receptor shedding on the activated CD8+ T cell. In this prior report, the ability of ADAM17 to function in trans was not tested directly but assumed by differences in ADAM17 expression on myeloid-derived suppressor cells and T cells [24]. Third, the activation threshold of TNFR2 shedding was similar to that of TNF-α expression, indicating that the CD8+ T cell would completely shed memTNFR2 when TCR signaling was strong enough to induce TNF-α production by that cell. Finally, inhibition of ADAM17-mediated shedding appeared to result in an autocrine interaction of memTNF-α with memTNFR2. Therefore, an important question that remains to be investigated is how expression and shedding of TNFR2 impact CD8+ T cell effector function. However, we were unable to address this in the current study by use of ADAM17−/− CD8+ T cells or broad-spectrum protease inhibitors, as both also inhibit TNF-α shedding. The ideal reagent to test these hypotheses would be a mouse that exclusively expresses noncleavable memTNFR2, which would allow one to dissect the impact of TNFR2 shedding from TNF-α shedding.
We also found that TNFR2 had an important role in regulating the availability of solTNF-α during influenza infection, as solTNFR2 levels were increased in the airways and serum after influenza infection in mice and TNFR2−/− or antibody blockade of TNFR2 enhanced solTNF-α levels in the airways. solTNFR2 production by activated influenza-specific CD8+ T cells was also capable of reducing the availability of solTNF-α produced by these cells in vitro. Moreover, solTNFR2 and solTNF-α were recruited to the immunologic synapse of activated CD8+ T cells, raising the possibility that solTNFR2 was shed concurrently with solTNF-α to limit TNF-α signaling in the target cell or leakage of solTNF-α from this junction. The regulation of solTNF-α levels during influenza infection is of critical importance, as we have demonstrated previously that ADAM17-mediated processing of TNF-α by influenza-specific CD8+ T cells was required for the development of severe and lethal lung injury in mice [16]. In humans, solTNF-α levels in nasal lavage samples have been shown to correlate with the onset and severity of disease symptoms in experimental [38, 39] and community-acquired [40, 41] influenza infection. As we observed elevated levels of solTNFR2 in humans after LAIV challenge, the extent by which solTNFR2 limits the availability of solTNF-α may have important implications for disease progression. Thus, future studies are required to understand the full consequence of TNFR2 expression and shedding in regulating solTNF-α levels during influenza infection and the impact this has on pulmonary pathology.
It is important to note that other than impacting our ability to detect solTNF-α levels (which we infer as reduced bioavailability), our study does not elucidate whether solTNFR2 is acting as an agonist or antagonist for solTNF-α signaling. Previous reports have demonstrated that solTNFR2 can reduce solTNF-α-mediated cytotoxicity in vitro, supporting an antagonistic role for solTNFR2 [13–15]. It has also been reported that solTNFR2 can function as an agonist, enhancing solTNF-α-mediated cell growth in vitro [15]. These studies often used supraphysiologic levels of solTNFR2 or recombinant TNFR2 to achieve these effects, and even though we observed increased solTNF-α levels following TNFR2 blockade in vitro and in vivo, likewise, high levels of recombinant TNFR2 were needed in our studies to reduce solTNF-α levels in vitro. Therefore, whether solTNFR2 acts as an agonist or antagonist in vivo remains unclear and may depend, in part, on the form of solTNFR2. Whereas dimeric solTNFR2 molecules have been shown to reduce serum solTNF-α levels following injection of LPS in mice, monomeric solTNFR2 molecules actually enhanced serum solTNF-α levels, indicating that the oligomeric status of the receptor may dictate its biologic function [42]. This may have important implications, as we show here that solTNFR2 levels are increased after LAIV challenge in humans, and increased levels of solTNFR2 have been shown to correlate with the severity of a number of diseases [18–20], including the extent of morbidity and pulmonary pathology during influenza infection in mice, as demonstrated here. Given the success of anti-TNF-α therapies in a variety of diseases, despite the presence of elevated levels of solTNFR2 during these diseases, it is tempting to speculate that solTNFR2 may function as a carrier of solTNF-α, prolonging TNF-α bioactivity, and thus, solTNFR2 may also represent an attractive therapeutic target. Alternatively, the majority of solTNFR2 may be functionally inactive or impaired and serve only as a surrogate for the extent of immune responses or severity of disease. However, expression of disease-relevant levels of human TNFR2 in mice results in multiorgan inflammation, independent of TNF-α [43], providing further support that specific antagonism of TNFR2 may be beneficial in limiting influenza immunopathology.
In conclusion, we have shown that TCR signaling in effector CD8+ T cells activates the MAPK/ERK pathway and induces TNFR2 shedding by ADAM17. With similar activation thresholds for TNF-α production and TNFR2 shedding, it seems likely that processing of TNFR2 by activated CD8+ T cells functions to modify the sensitivity of the activated cell to TNF-α. Meanwhile, the shed receptor may function to regulate the bioavailability of solTNF-α. Future studies of TNFR2 shedding by CD8+ T cells will be aimed at understanding its role in T cell homeostasis and its impact on TNF-α-mediated pathology.
AUTHORSHIP
M.P.D. participated in the experimental design, performed the experiments, interpreted the data, and wrote the manuscript. K.H.E., P.F.W., and E.B.T. participated in experimental design, data interpretation, and manuscript revision. R.I.E. participated in experimental design, data interpretation, and writing of the manuscript.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health(NIH) National Institute of Allergy and Infectious Diseases Grants T32A107363 (to M.P.D.) and U19AI 083024 (to R.I.E.), NIH National Heart, Lung, and Blood Institute Grant R01HL122309 ( to E.B.T.), and NIH Contract HHSN272200900026C (to P.F.W.). The authors gratefully acknowledge the generous support of the Brody Idiopathic Pulmonary Fibrosis Research Fund and the Ira Jerome Brody ʼ44 Memorial Fund. The authors thank Dr. Mark Schneider for critical reading of this manuscript and acknowledge the NIH Tetramer Core Facility (Contract HHSN272201300006C) for provision of APC-conjugated NP366–374 tetramers. The authors also acknowledge Kenneth Orndorff of the Microscopy Shared Resource of Dartmouth-Hitchcock Norris Cotton Cancer Center for assistance with cellular imaging and DartLab: Immunoassay and Flow Cytometry Shared Resource at the Geisel School of Medicine at Dartmouth.
Glossary
- −/−
deficient/knockout
- ADAM17
a disintegrin and metalloproteinase 17
- APC
allophycocyanin
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- CD62L
cluster of differentiation 62 ligand
- CtxB
cholera toxin B
- LAIV
live-attenuated influenza vaccine
- mem
transmembrane
- MLN
mediastinal lymph node
- NP
nucleoprotein
- sol
soluble
- TAPI
TNF-α protease inhibitor
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Aggarwal B. B. (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756. [DOI] [PubMed] [Google Scholar]
- 2.Grell M., Douni E., Wajant H., Löhden M., Clauss M., Maxeiner B., Georgopoulos S., Lesslauer W., Kollias G., Pfizenmaier K., Scheurich P. (1995) The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83, 793–802. [DOI] [PubMed] [Google Scholar]
- 3.Liu J., Zhao M. Q., Xu L., Ramana C. V., Declercq W., Vandenabeele P., Enelow R. I. (2005) Requirement for tumor necrosis factor-receptor 2 in alveolar chemokine expression depends upon the form of the ligand. Am. J. Respir. Cell Mol. Biol. 33, 463–469. [DOI] [PubMed] [Google Scholar]
- 4.Richter M. V., Topham D. J. (2007) The alpha1beta1 integrin and TNF receptor II protect airway CD8+ effector T cells from apoptosis during influenza infection. J. Immunol. 179, 5054–5063. [DOI] [PubMed] [Google Scholar]
- 5.Ware C. F., Crowe P. D., Vanarsdale T. L., Andrews J. L., Grayson M. H., Jerzy R., Smith C. A., Goodwin R. G. (1991) Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type I TNF receptor during activation of resting and effector T cells. J. Immunol. 147, 4229–4238. [PubMed] [Google Scholar]
- 6.Cope A. P., Aderka D., Wallach D., Kahan M., Chu N. R., Brennan F. M., Feldmann M. (1995) Soluble TNF receptor production by activated T lymphocytes: differential effects of acute and chronic exposure to TNF. Immunology 84, 21–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Kim E. Y., Teh H. S. (2001) TNF type 2 receptor (p75) lowers the threshold of T cell activation. J. Immunol. 167, 6812–6820. [DOI] [PubMed] [Google Scholar]
- 8.Kim E. Y., Priatel J. J., Teh S. J., Teh H. S. (2006) TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J. Immunol. 176, 1026–1035. [DOI] [PubMed] [Google Scholar]
- 9.Aspalter R. M., Eibl M. M., Wolf H. M. (2003) Regulation of TCR-mediated T cell activation by TNF-RII. J. Leukoc. Biol. 74, 572–582. [DOI] [PubMed] [Google Scholar]
- 10.Kim E. Y., Teh H. S. (2004) Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J. Immunol. 173, 4500–4509. [DOI] [PubMed] [Google Scholar]
- 11.Kim E. Y., Teh S. J., Yang J., Chow M. T., Teh H. S. (2009) TNFR2-deficient memory CD8 T cells provide superior protection against tumor cell growth. J. Immunol. 183, 6051–6057. [DOI] [PubMed] [Google Scholar]
- 12.Crowe P. D., Walter B. N., Mohler K. M., Otten-Evans C., Black R. A., Ware C. F. (1995) A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J. Exp. Med. 181, 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelmann H., Novick D., Wallach D. (1990) Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J. Biol. Chem. 265, 1531–1536. [PubMed] [Google Scholar]
- 14.Balcewicz-Sablinska M. K., Keane J., Kornfeld H., Remold H. G. (1998) Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 161, 2636–2641. [PubMed] [Google Scholar]
- 15.Aderka D., Engelmann H., Maor Y., Brakebusch C., Wallach D. (1992) Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J. Exp. Med. 175, 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBerge M. P., Ely K. H., Cheng G. S., Enelow R. I. (2013) ADAM17-mediated processing of TNF-α expressed by antiviral effector CD8+ T cells is required for severe T-cell-mediated lung injury. PLoS ONE 8, e79340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., Matsuoka M., Cantor H., Homer R., Enelow R. I. (2008) Cutting edge: engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J. Immunol. 180, 25–29. [DOI] [PubMed] [Google Scholar]
- 18.Cope A. P., Aderka D., Doherty M., Engelmann H., Gibbons D., Jones A. C., Brennan F. M., Maini R. N., Wallach D., Feldmann M. (1992) Increased levels of soluble tumor necrosis factor receptors in the sera and synovial fluid of patients with rheumatic diseases. Arthritis Rheum. 35, 1160–1169. [DOI] [PubMed] [Google Scholar]
- 19.Aderka D., Wysenbeek A., Engelmann H., Cope A. P., Brennan F., Molad Y., Hornik V., Levo Y., Maini R. N., Feldmann M., Wallach D. (1993) Correlation between serum levels of soluble tumor necrosis factor receptor and disease activity in systemic lupus erythematosus. Arthritis Rheum. 36, 1111–1120. [DOI] [PubMed] [Google Scholar]
- 20.Marinos G., Naoumov N. V., Rossol S., Torre F., Wong P. Y., Gallati H., Portmann B., Williams R. (1995) Tumor necrosis factor receptors in patients with chronic hepatitis B virus infection. Gastroenterology 108, 1453–1463. [DOI] [PubMed] [Google Scholar]
- 21.Peschon J. J. S. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., Cerretti D. P., Paxton R. J., March C. J., Black R. A. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284. [DOI] [PubMed] [Google Scholar]
- 22.Bell J. H. H. A., Herrera A. H., Li Y., Walcheck B. (2007) Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J. Leukoc. Biol. 82, 173–176. [DOI] [PubMed] [Google Scholar]
- 23.Briso E. M. D. O., Dienz O., Rincon M. (2008) Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J. Immunol. 180, 7102–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanson E. M. C. V., Clements V. K., Sinha P., Ilkovitch D., Ostrand-Rosenberg S. (2009) Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J. Immunol. 183, 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soond S. M. E. B., Everson B., Riches D. W., Murphy G. (2005) ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J. Cell Sci. 118, 2371–2380. [DOI] [PubMed] [Google Scholar]
- 26.Díaz-Rodríguez E., Montero J. C., Esparís-Ogando A., Yuste L., Pandiella A. (2002) Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol. Biol. Cell 13, 2031–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tellier E., Canault M., Rebsomen L., Bonardo B., Juhan-Vague I., Nalbone G., Peiretti F. (2006) The shedding activity of ADAM17 is sequestered in lipid rafts. Exp. Cell Res. 312, 3969–3980. [DOI] [PubMed] [Google Scholar]
- 28.Von Tresckow B., Kallen K. J., von Strandmann E. P., Borchmann P., Lange H., Engert A., Hansen H. P. (2004) Depletion of cellular cholesterol and lipid rafts increases shedding of CD30. J. Immunol. 172, 4324–4331. [DOI] [PubMed] [Google Scholar]
- 29.Xu P., Liu J., Sakaki-Yumoto M., Derynck R. (2012) TACE activation by MAPK-mediated regulation of cell surface dimerization and TIMP3 association. Sci. Signal. 5, ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Gall S. M., Maretzky T., Issuree P. D., Niu X. D., Reiss K., Saftig P., Khokha R., Lundell D., Blobel C. P. (2010) ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J. Cell Sci. 123, 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu P., Derynck R. (2010) Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol. Cell 37, 551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinclair L. V. F. D., Finlay D., Feijoo C., Cornish G. H., Gray A., Ager A., Okkenhaug K., Hagenbeek T. J., Spits H., Cantrell D. A. (2008) Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat. Immunol. 9, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilyushina N. A., Haynes B. C., Hoen A. G., Khalenkov A. M., Housman M. L., Brown E. P., Ackerman M. E., Treanor J. J., Luke C. J., Subbarao K., Wright P. F. (2015) Live attenuated and inactivated influenza vaccines in children. J. Infect. Dis. 211, 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner S. J., La Gruta N. L., Stambas J., Diaz G., Doherty P. C. (2004) Differential tumor necrosis factor receptor 2-mediated editing of virus-specific CD8+ effector T cells. Proc. Natl. Acad. Sci. USA 101, 3545–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faroudi M., Utzny C., Salio M., Cerundolo V., Guiraud M., Müller S., Valitutti S. (2003) Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc. Natl. Acad. Sci. USA 100, 14145–14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Al-Lamki R. S., Zhang H., Kirkiles-Smith N., Gaeta M. L., Thiru S., Pober J. S., Bradley J. R. (2003) Histamine antagonizes tumor necrosis factor (TNF) signaling by stimulating TNF receptor shedding from the cell surface and Golgi storage pool. J. Biol. Chem. 278, 21751–21760. [DOI] [PubMed] [Google Scholar]
- 37.Heaney M. L., Golde D. W. (1996) Soluble cytokine receptors. Blood 87, 847–857. [PubMed] [Google Scholar]
- 38.Hayden F. G., Fritz R., Lobo M. C., Alvord W., Strober W., Straus S. E. (1998) Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J. Clin. Invest. 101, 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fritz R. S., Hayden F. G., Calfee D. P., Cass L. M., Peng A. W., Alvord W. G., Strober W., Straus S. E. (1999) Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J. Infect. Dis. 180, 586–593. [DOI] [PubMed] [Google Scholar]
- 40.Kaiser L., Fritz R. S., Straus S. E., Gubareva L., Hayden F. G. (2001) Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J. Med. Virol. 64, 262–268. [DOI] [PubMed] [Google Scholar]
- 41.Hagau N., Slavcovici A., Gonganau D. N., Oltean S., Dirzu D. S., Brezoszki E. S., Maxim M., Ciuce C., Mlesnite M., Gavrus R. L., Laslo C., Hagau R., Petrescu M., Studnicska D. M. (2010) Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit. Care 14, R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohler K. M., Torrance D. S., Smith C. A., Goodwin R. G., Stremler K. E., Fung V. P., Madani H., Widmer M. B. (1993) Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J. Immunol. 151, 1548–1561. [PubMed] [Google Scholar]
- 43.Douni E., Kollias G. (1998) A critical role of the p75 tumor necrosis factor receptor (p75TNF-R) in organ inflammation independent of TNF, lymphotoxin alpha, or the p55TNF-R. J. Exp. Med. 188, 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.