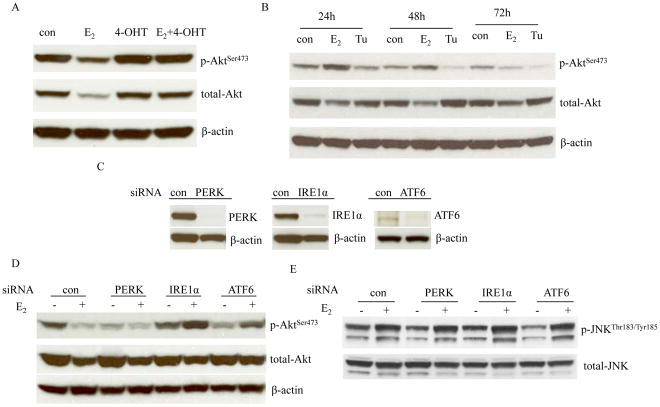
Figure 6. Regulation of Akt by endoplasmic reticulum stress.
(A) 4-OHT prevented the degradation of Akt by E2. MCF-7:5C cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), 4-OHT (10−6 mol/L), and 4-OHT (10−6 mol/L) plus E2 (10−9 mol/L) for 72 hours. Cell lysates were harvested. p-Akt and total Akt were examined by Western blotting. β-actin was measured as loading control. (B) Effects of Tunicamycin on Akt phosphorylation. MCF-7:5C cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), and Tunicamycin (10−5 mol/L) for different time points indicated. p-Akt and total-Akt were examined by Western blotting. β-actin was measured as loading control. (C) Knockdown of three sensors of endoplasmic reticulum stress by siRNAs. MCF-7:5C cells were transfected with scrambled, PERK, IRE1α, and ATF6 siRNA for 72 hours. Respective proteins were examined by Western blotting. β-actin was measured as loading control. (D) Effects on Akt after knockdown of three sensors. MCF-7:5C cells were transfected with specific siRNAs as above for 72 hours. Then, cells were treated with or without E2 for 72 hours. p-Akt and total-Akt were examined by Western blotting. β-actin was measured as loading control. (E) Effects on JNK phosphorylation after knockdown of three sensors. Cell lysates were the same as in (D). p-JNK and total-JNK were examined by Western blotting.