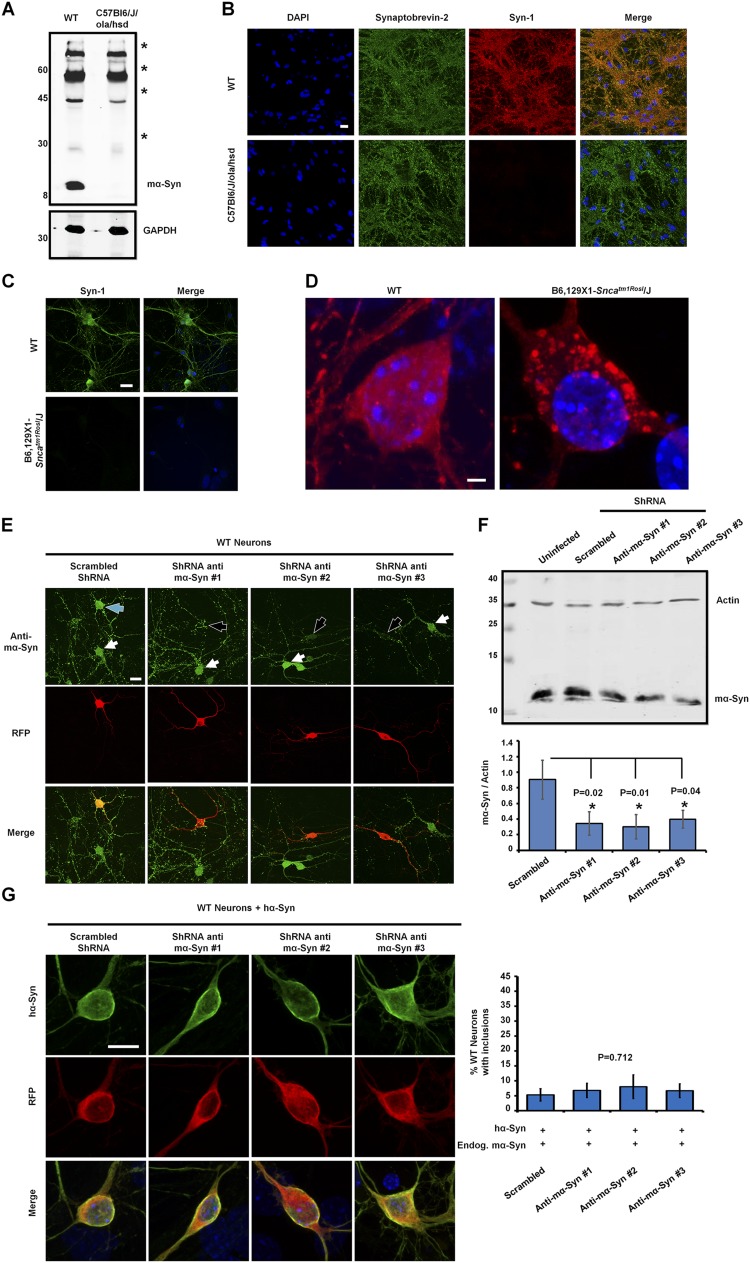
Fig. S1.
The total abolition of mα-Syn expression is necessary to promote the formation of hα-Syn inclusions. (A and B) The absence of mα-Syn expression in C57Bl6/J/ola/hsd mice was established by Western blot analysis of brain homogenates (A) and by immunocytochemistry of primary neurons (B), which showed no mα-Syn staining in synaptic terminals of KO neurons. (The asterisks in A indicate nonspecific signals that are detected equally in WT and SNCA−/− brains.) (C and D) Similarly, immunocytochemistry established the loss of mα-Syn expression in primary neurons derived from B6,129 × 1-Sncatm1Rosl/J mice (C) and revealed that overexpressed hα-Syn forms inclusions when expressed in B6,129 × 1-Sncatm1Rosl/J primary neurons (D). (E) Immunocytochemistry using an anti–mα-Syn–specific antibody (green) shows that WT neurons transfected with plasmids encoding anti–mα-Syn ShRNA (having RFP as a marker of transfection; black arrows) show reduced levels of mα-Syn as compared with nontransfected neurons (white arrows). In contrast, neurons expressing scrambled shRNA (blue arrow) do not show any change in mα-Syn levels. (F) Biochemical analysis of WT neurons infected with lentiviruses shows that the levels of endogenous mα-Syn are decreased significantly with anti–mα-Syn shRNA expression as compared with the expression of scrambled shRNA (n = 3 independent experiments). One-way ANOVA and Scheffé post hoc analysis were applied to obtain P values. (G) WT neurons coexpressing hα-Syn with anti–mα-Syn shRNA (having RFP as a marker of transfection) exhibit diffuse localization of hα-Syn similar to that seen when scrambled shRNA is expressed. Quantification of the formation of inclusions (>1 µm) in 25 neurons per condition (mean ± SD, n = 3 independent experiments) reveals no significant differences across conditions (P = 0.712, one-way ANOVA and Scheffé post hoc analysis). (Scale bars: 20 µm in B, C, and E, 2 µm in D, and 10 µm in G.)