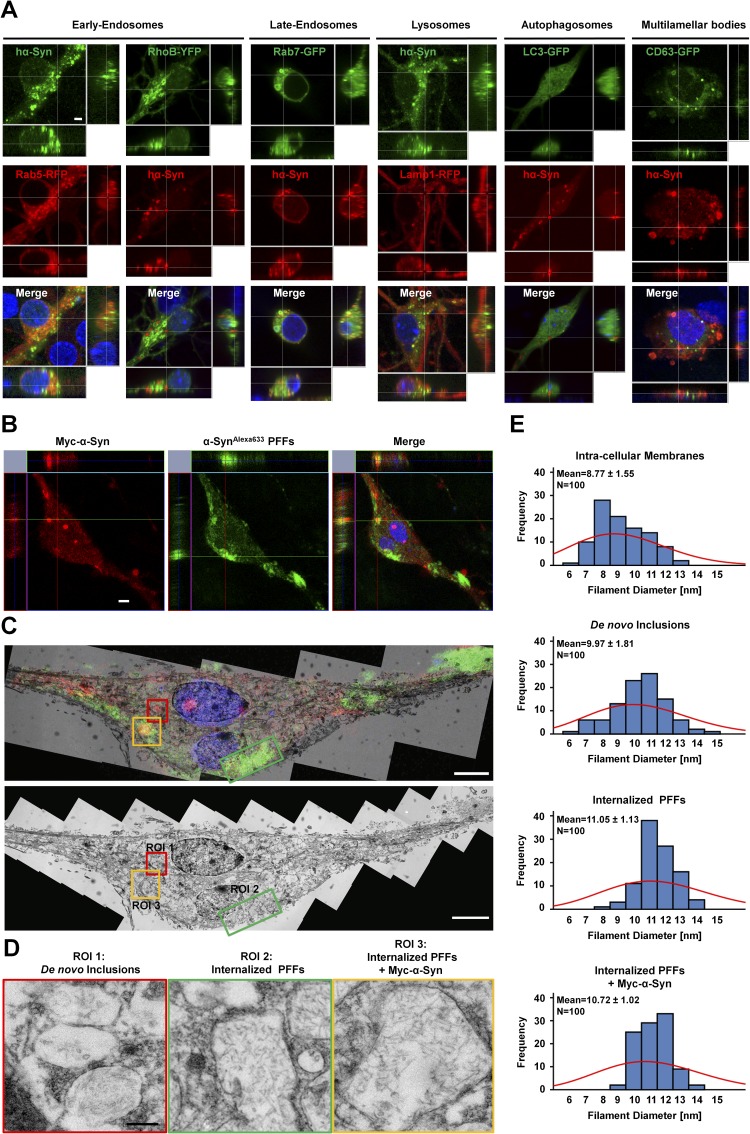
Fig. S4.
Characterization of hα-Syn aggregates by immunofluorescence and electron microscopy. (A) Immunocytochemistry shows partial weak colocalization of hα-Syn inclusions with cotransfected markers of late endosomes (Rab7-GFP) and lysosomes (Lamp1-RFP), early endosomes (RhoB-YFP and Rab5-RFP), auto-phagosomes (LC3-GFP), and multilamellar bodies (GFP-CD63). (Scale bar: 2 µm.) (B) SNCA−/− neurons were transfected to express Myc–hα-Syn and 24 h later were treated with hα-SynAlexa633 PFFs for an additional 24 h. Immunocytochemistry using an anti-Myc antibody was performed; then stained neurons were imaged by confocal microscopy. hα-SynAlexa633 PFFs are shown in green to allow better visualization in following panels. (C) The two adjacent neurons imaged by fluorescent microscopy were reprocessed and imaged by TEM. (Upper) To correlate structures observed by ssTEM and fluorescence, single-plane images were superimposed. (Lower) A single-plane TEM image is shown with designated ROIs that comprise de novo inclusions composed of Myc–hα-Syn alone (ROI1), internalized hα-SynAlexa633 PFFs alone (ROI2), or mixtures of hα-SynAlexa633 PFFs and Myc–hα-Syn (ROI3). (D) High-resolution images of inclusions in ROI1, ROI2, and ROI3. (E) Quantification of filament diameter (n = 100 per condition) shows that the overall distribution of membrane diameters is different from that of filaments observed in de novo inclusions and from that of internalized PFFs (± Myc–hα-Syn). A frequency histogram with Poisson distribution (red) is shown, together with mean ± SD values. (Scale bars: 5 µm in B and C and 250 nm in D.)