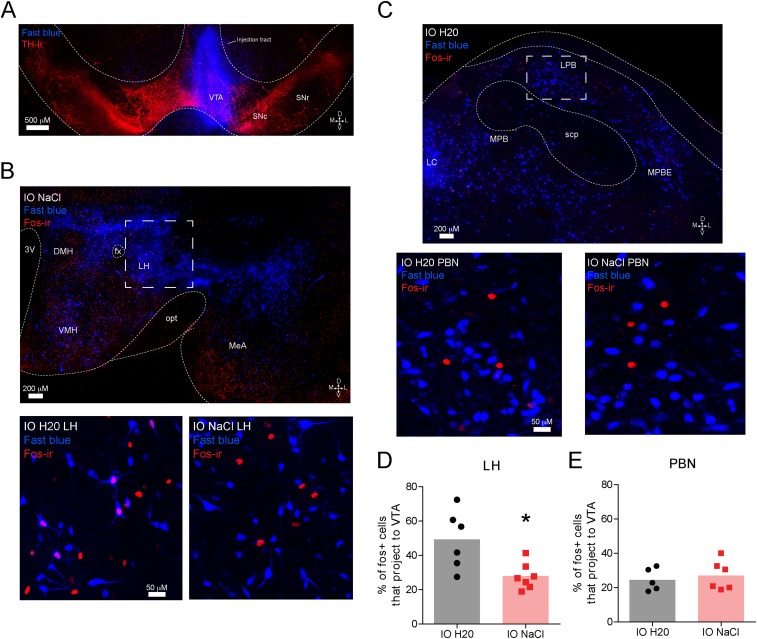
Fig. S3.
Sodium-depleted rats that received intraoral infusions of NaCl have less Fos immunoreactivity in VTA projecting neurons from the LH, but not the PBN, compared with sodium-depleted rats that received intraoral distilled H2O (related to Fig. 1). Interestingly, we observed a significant decrease in the magnitude of dopamine release evoked by intraoral NaCl across the session in sodium-depleted rats (Fig. S2). This result suggested that the input (or inputs) providing NaCl-related information to VTA dopamine neurons becomes quiescent as rats recovered lost sodium. To investigate this possibility, we injected the VTA with Fast Blue [250 nL, 2% (vol/vol) in distilled water], a fluorescent retrograde tracer (n = 7 rats). Ten days later, all rats were sodium-depleted for 24 h. The behavioral session was identical to the unconditioned intraoral infusion session used in the voltammetry experiments presented in Fig. 1. Sodium-depleted rats received 30 intraoral infusions of either 0.45 M NaCl (n = 4) or distilled water (n = 3). Given that the magnitude of NaCl-evoked dopamine release decayed across the infusion session (Fig. S2C), we reasoned that a VTA input capable of driving dopamine responses to NaCl might be differentially activated by sodium depletion with (intraoral NaCl) or without (intraoral distilled water) partial repletion. One hour following sodium depletion, all rats were perfused and brains were stored in cryoprotectant in preparation for sectioning, immunohistochemistry, and confocal microscopy. We focused our analyses on the PBN and the LH because both are critical for sodium appetite (52) and are positioned at different stages of integrating visceral and gustatory signals. (A) Confocal image (20×) of a representative VTA injection site depicting tyrosine hydroxylase (TH) immunoreactivity (red) and the injection site of Fast Blue (blue). D, dorsal; L, lateral; M, medial; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; V, ventral. (B, Top) Confocal image (20×) at the level of the hypothalamus in a sodium-depleted rat that received intraoral NaCl. The image depicts retrograde labeling of VTA projecting neurons (blue) and Fos immunoreactivity (red). DMH, dorsomedial hypothalamus; fx, fornix; IO, intraoral; MeA, medial amygdala; opt, optic tract; 3V, third ventricle; VMH, ventromedial hypothalamus. The dashed box indicates the approximate LH region of interest (ROI) used for quantification in D. (B, Bottom) Confocal image (20×) zoomed in to the LH ROI in a representative sodium-depleted rat that received intraoral H2O (Left) or intraoral NaCl (Right). Note the absence of colabeling following intraoral NaCl. (C, Top) Confocal image (20×) at the level of the lateral PBN from a rat that received intraoral H2O. The image depicts retrograde labeling of VTA projecting neurons (blue) and Fos immunoreactivity (red). LC, locus coeruleus; MPB, medial parabrachial region; MPBE, medial parabrachial external region; scp, superior cerebellar peduncles. The dashed box indicates the approximate LPB ROI used for quantification in E. (C, Bottom) Confocal image (20×) zoomed in on the LPB ROI in a representative sodium-deficient rat that received intraoral H20 (Left) or intraoral NaCl (Right). (D) Graph depicts the percentage of Fos-positive LH neurons that project to the VTA in sodium-depleted rats that received intraoral H2O (black, n = 3 rats, two LH slices per rat) vs. intraoral NaCl (red, n = 4 rats, two LH slices from three rats, one LH slice from one rat). Given the variability in tracer uptake and transport, as well as Ab staining, each slice was treated as an independent observation. Rats that received intraoral NaCl had a significantly smaller proportion of Fos-positive cells that projected to the VTA compared with rats that received intraoral H2O (unpaired t test: t11 = 3.0, *P < 0.05). The shaded bar in the background indicates the mean; data from individual LH slides are represented by individual dots. (E) Same as in D but for LPB neurons that project to the VTA [intraoral H2O (black, n = 3 rats, two LPB slices from two rats, one LPB slice from one rat) vs. intraoral NaCl (red, n = 4 rats, three PBN slices from three rats, one PBN slice from one rat)]. One slice was removed from each group due to poor hind-brain tracer expression. No differences were observed in the LPB (unpaired t test: t9 = 0.54, P > 0.05). Thus, VTA LH neurons may drive phasic dopamine responses to NaCl in sodium-depleted rats, and this drive may weaken as repletion occurs. Moreover, these data indicate that the transformation in NaCl encoding relevant to dopamine signaling likely occurs after initial stages of central visceral and gustatory processing because no differences were observed in the PBN. Interestingly, LH lesions disrupt sodium consumption in sodium-depleted animals, even after animals have recovered from the adipsia and anorexia typically induced by such lesions (53). These results raise the possibility that the deficit in sodium consumption following LH lesions may result from the elimination of LH drive to VTA dopamine neurons.