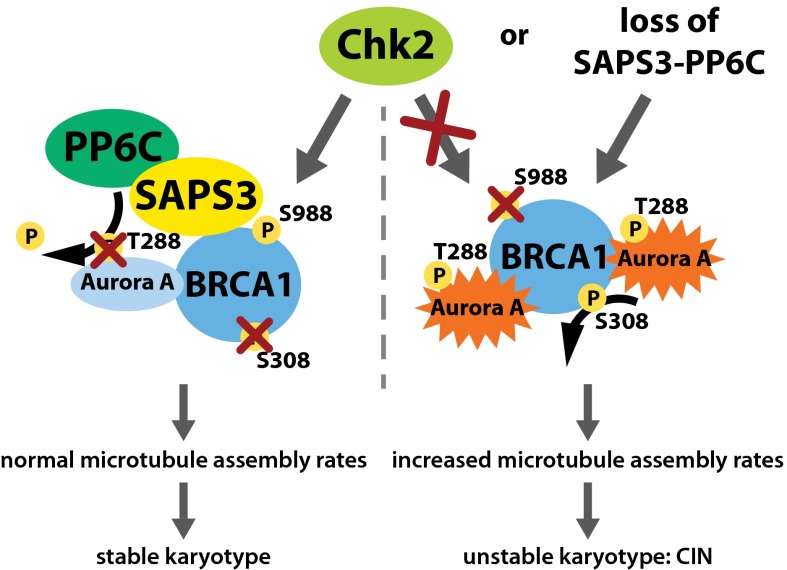
Fig. 6.
Model summarizing the mitotic regulation of BRCA1 required for the maintenance of proper microtubule assembly and chromosomal stability. Aurora-A can inactivate the mitotic function of BRCA1 by direct phosphorylation on S308, resulting in increased microtubule assembly, chromosome missegregation, and CIN. During normal mitosis, however, BRCA1 is phosphorylated by Chk2 on S988, triggering the recruitment of the SAPS3-PP6 phosphatase complex to BRCA1 and thereby leading to dephosphorylation of Aurora-A restraining Brca1-bound Aurora-A activity. The limited Aurora-A activity, in turn, limits BRCA1 phosphorylation on S308 and thus, its inactivation. In cancer cells, the overexpression of oncogenic AURKA or the loss of the tumor suppressors CHK2 or PP6 leads to failure of this fail-safe mechanism and promotes the Aurora-A–dependent inactivation of BRCA1, resulting in CIN and aneuploidy as seen upon loss of BRCA1.