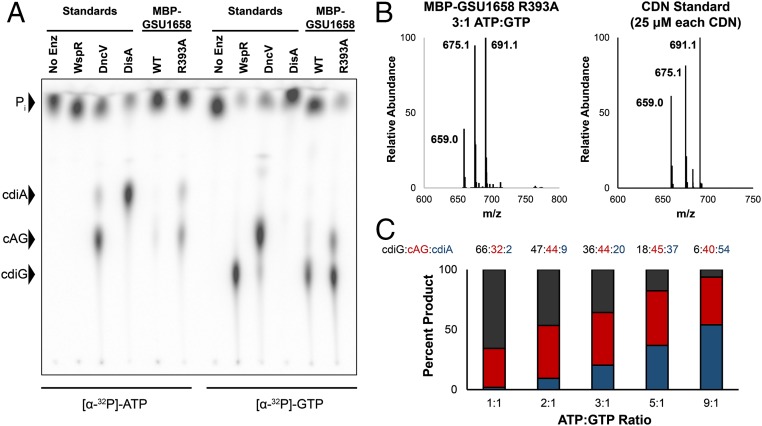
Fig. 2.
GSU1658 produces different cyclic dinucleotides depending on ATP-to-GTP ratios. (A) Cellulose TLC analysis of radiolabeled products from enzymatic reactions with 1:1 ATP-to-GTP substrates in excess and doped with trace amounts of α-32P-labeled ATP or α-32P-labeled GTP. Before loading, reactions were quenched with alkaline phosphatase to digest unreacted nucleotides, resulting in production of inorganic phosphate (Pi). Residue R393 is located in the putative I-site. (B) Representative LC/MS analysis of an enzymatic assay performed with MBP-GSU1658 R393A at 3:1 ratio of ATP to GTP in comparison with a standard containing all three product CDNs at equal concentrations. Shown is the MS spectrum from integrating the retention time region containing all three cyclic dinucleotides. Expected masses are for cdiG (m/z = 691), cAG (m/z = 675), and cdiA (m/z = 659). (C) Analysis of product ratios for MBP-GSU1658 R393A at different ATP-to-GTP ratios based on LC/MS analysis and comparison with CDN standard to account for different ionization efficiencies. Average values of two replicate runs are shown.