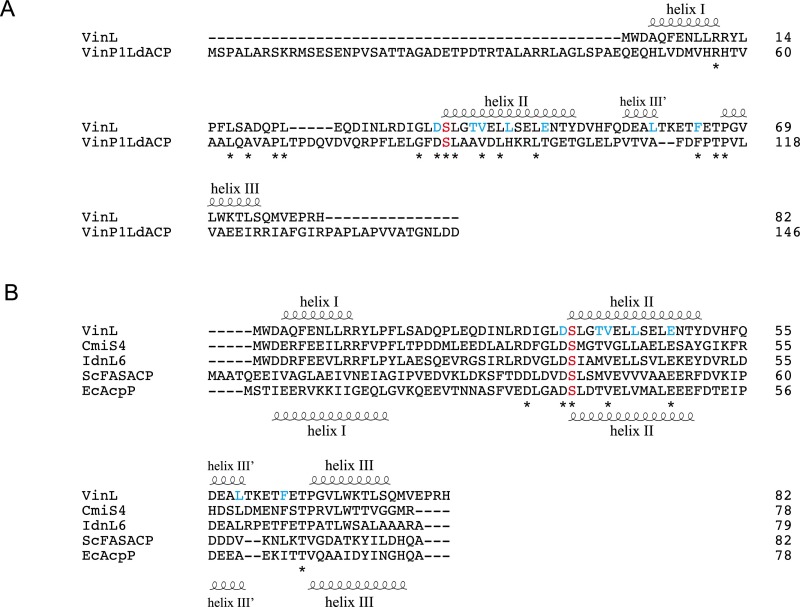
Fig. S6.
Amino acid sequence alignment of VinL with other ACPs. The secondary structural elements of VinL and EcAcpP are indicated above and below the sequences, respectively. A conserved Ser residue for the attachment of 4′-phosphopantetheine arm is shown as red. VinL residues important for the interaction with VinK are shown as cyan. (A) Amino acid sequence comparison of VinL with VinP1LdACP. (B) Amino acid sequence alignment of VinL with other VinL-like proteins involved in the macrolactam biosynthesis (CmiS4 from Streptomyces sp. MJ635-86F5 and IdnL6 from Streptomyces sp. ML694-90F3) and other related ACP proteins (ScFASACP from S. coelicolor and EcAcpP from E. coli).