ABSTRACT
HIV-associated lipodystrophy commonly presents with fat loss in the face, buttocks, arms and legs, hypocomplementaemia, glomerulonephritis and autoimmune disorders. The exact mechanism of HIV-associated lipodystrophy is not fully elucidated. There is evidence indicating that it can be caused by both antiretroviral medications and HIV infection in the absence of antiretroviral medication. Lipodystrophy seems to be mainly due to HIV-1 protease inhibitors. Interference with lipid metabolism is postulated as pathophysiology. Also, the development of lipodystrophy is associated with specific nucleoside reverse transcriptase inhibitors (NRTI). Mitochondrial toxicity is postulated to be involved in the pathogenesis associated with NRTI. Here, we analyse the side effects and examine the impact of the highly active antiretroviral therapy (HAART) regimen including raltegravir, lamivudine, darunavir and ritonavir in an HIV-1 infected patient with severe lipodystrophy after six years of antiretroviral therapy.
Keywords: Darunavir, HIV-1 infection, lamivudine, raltegravir, ritonavir, severe lipodystrophy
RESUMEN
La lipodistrofia asociada al VIH se presenta comúnmente con pérdida de grasa en la cara, nalgas, brazos y piernas, hipocomplementemia, glomerulonefritis y trastornos autoinmunes. El mecanismo exacto de la lipodistrofia asociada con el VIH no está totalmente aclarado. Evidencias indican que puede ser causada por medicamentos antirretrovirales y la infección por VIH en ausencia de tratamiento antirretroviral. La lipodistrofia parece ser principalmente debida a los inhibidores de proteasa de HIV-1. La interferencia con el metabolismo de los lípidos se postula como fisiopatología. Además, el desarrollo de la lipodistrofia se asocia con inhibidores específicos de la transcriptasa reversa análogos de nucleósidos (ITRN). Se postulada que la toxicidad mitocondrial está involucrada en la patogénesis asociada con ITRN. En el presente trabajo analizamos los efectos secundarios, y examinamos el impacto del régimen de la terapia antirretroviral de gran actividad (TARGA) incluyendo raltegravir, lamivudina, darunavir y ritonavir en un paciente infectado con VIH-1 con lipodistrofia severa después de seis años de terapia antirretroviral.
INTRODUCTION
The first case of lipodystrophy was described by Mitchell in 1886 (1) and later cases were described by Barraquer in 1907 and Simons in 1911. The onset is usually insidious with the slow, progressive disappearance of subcutaneous fat involving the upper half of the body. The predictive progression of the disease from the face to the neck, upper extremities, and trunk (sparing the buttocks and lower limbs) is characteristic. HIVassociated lipodystrophy commonly presents with fat loss in the face, buttocks, arms and legs, hypocomplementaemia, glomerulonephritis, and autoimmune disorders.
The exact mechanism of HIV-associated lipodystrophy is not fully elucidated. There is evidence indicating both that it can be caused by antiretroviral medications and HIV infection in the absence of antiretroviral medication.
Lipodystrophy seems to be mainly due to HIV-1 protease inhibitors. Interference with lipid metabolism is postulated as pathophysiology. Also, the development of lipodystrophy is associated with specific nucleoside reverse transcriptase inhibitors (NRTI). Mitochondrial toxicity is postulated to be involved in the pathogenesis associated with NRTI.
Here, we examine the impact of highly active antiretroviral therapy (HAART) regiment including raltegravir, lamivudine, darunavir, ritonavir in an HIV-1 infected patient with severe lipodystrophy.
CASE REPORT
The patient is a male Italian, aged 60 years old with a history of homosexuality since 1994. He was first diagnosed with HIV-1 infection in February 1996. He was HBV/HCV negative and had no other sexually transmitted diseases (STDs). His multidrug-resistance profile at baseline was (test TRUGENE HIV-1): reverse transcriptase mutations M41L, K65R, K70R, V75I, F77L, Q151M, T215Y, K219E; protease inhibitor mutations L10V, K20R, L33F, M36I, M46I, G48V, I54T, L63P, A71V, V77I, V82I and I84V.
At the time of admission (March 2008), the patient's HAART combination was raltegravir, lamivudine, darunavir and ritonavir; CD4 T-cell count was 214 cell/mm3 and plasma HIV RNA concentration was undetectable (detection limit 50 copies/mL). Aspartate transaminase (AST) was 39 IU/L (reference range, 1–36 IU/L), alanine aminotransaminase (ALT) 29 IU/L (reference range, 1–36 IU/L), total bilirubin 1.0 mg/dL, triglycerides 201 mg/dL, total cholesterol 245 mg/dL, high-density lipoprotein (HDL) cholesterol 59 mg/dL, creatinine 1.10 mg and glycaemia 90 mg/dL without history of insulin resistance, hypocomplementaemia, glomerulonephritis and autoimmune disorders.
At entry, he was moderately active but did not exercise regularly. He gained 12 kg between January 2007 and September 2009 but noted loss of fat and bulging veins in his arms and legs, along with an increase in abdominal girth, which required an increase in his pants size, and an increase in breast size.
The diagnosis of severe mixed lipodystrophy was based on clinical signs (peripheral lipoatrophy: greater subcutaneous fat loss in arms, legs, buttocks, or face which were scored as severe) plus increased fat accumulation in the abdomen or breasts, with additional fat at the back of the neck. Objective parameters (fasting lipids and glucose evaluation), anthropometric measures (body mass index [BMI], waist to hip ratio [WHR]), dermal and subcutaneous thickness of both cheeks measured with a high frequency ultrasound transducer (7.5 MHz), dual-energy X-ray absorptiometry (DEXA) evaluation of total body, trunk, and leg fat and lean mass, abdominal computed tomography (CT) scan for visceral (VAT) and subcutaneous adipose tissue (SAT) were routinely collected at the time of first encounter of the patient, before providing any medical or surgical treatment.
The degree of lipoatrophy, diffuse fat accumulation, or lipomatosis at every site on the body was rated as absent (score of 0), mild (noticeable on close inspection, score of 1), moderate [readily noticeable by patient or physician, score of 2], or severe [readily noticeable to a casual observer, score of 3] (2). During subsequent follow-up, the patient maintained good clinical state and he adhered to the HAART regimen.
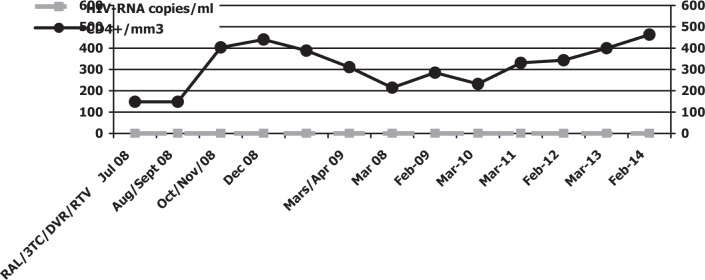
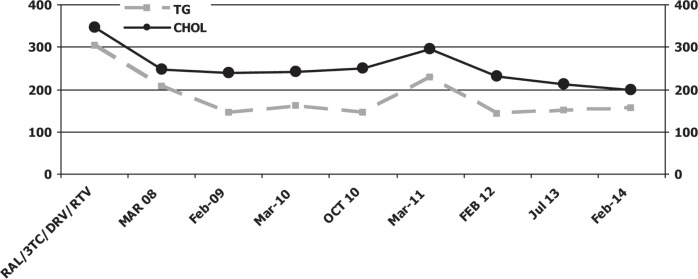
In October 2009, biochemical, haematological and viro-immunological parameters demonstrated a good response to drug therapy without side effects: CD4 T-cell count of 258 cell/mm3 and undetectable plasma HIV RNA concentration (detection limit 50 copies/mL), AST 30 IU/L, ALT 25 IU/L, total bilirubin 0.8 mg/dL, triglycerides 198 mg/dL, total cholesterol 200 mg/dL, HDL cholesterol 50 mg/dL, creatinine 1.10 mg and glycaemia 95 mg/dL. Clinical and objective signs of lipodystrophy had decreased. Currently, after five years of therapy with raltegravir, lamivudine, ritonavir and darunavir, laboratory and objective examinations reveal important reduction signs of mixed lipodystrophy without any interruption of antiretroviral therapy containing protease inhibitors as backbone (Figs. 1, 2).
Fig. 1. HIV-RNA viral load, CD4+ cell count and antiretroviral treatment.
RAL: raltegravir; 3TC: lamivudine; DRV: darunavir; RTV: ritonavir
Fig. 2. Serum triglyceride and cholesterol levels.
RAL: raltegravir; 3TC: lamivudine; DRV: darunavir; RTV: ritonavir
In February 2014, CD4 T-cell count was 370 cell/mm3 and plasma HIV RNA concentration was undetectable (detection limit 50 copies/mL), AST 35 IU/L, ALT 26 IU/L, total bilirubin 0.5 mg/dL, triglycerides 190 mg/dL, total cholesterol 200 mg/dL, HDL cholesterol 50 mg/dL, creatinine 1.0 mg, glycaemia 90 mg/dL and bilirubin 0.8 mg/dL. Clinical and objective signs of lipodystrophy had decreased. Surgery (excision or liposuction) was not performed on the patient because his severe fat accumulation was reduced without the interruption of protease inhibitors.
DISCUSSION
Lipodystrophy is a psychologically catastrophic and physiologically dangerous complication of treatment of HIV infection (3). Lipodystrophies can be a possible side effect of antiretroviral drugs. Lipodystrophies manifest as lipid redistribution, with excess, or lack of, fat in various regions of the body. These include, but are not limited to, having sunken cheeks and/or “humps” on the back or back of the neck (also referred to as buffalo hump) which is also caused by excess cortisol. Lipodystrophy can be caused by metabolic abnormalities due to genetic issues. These are often characterized by insulin resistance and are associated with the metabolic syndrome. On the other hand, there is evidence that HIV-1 infection on its own contributes to the development of the lipodystrophic phenotype by interfering with some key genes of adipocyte differentiation and mitochondrial function in patients who have not received antiretroviral treatment (4).
To date, no therapy for lipodystrophy is recognized as safe or effective, despite intensive research. Most of this research evaluated alterations of HAART regimens or administration of metabolically active substances, such as recombinant growth hormone, testosterone, or hypoglycaemic agents, such as metformin or the thioglitazones. However, these treatments are complex and expensive, and they are associated with many side effects.
The patient never stopped antiretroviral therapy and therefore did not develop drugs resistance. To our knowledge, this report is the first to show that with a slight diet change and exercise, patients are capable of reversing much of the metabolic and body composition change seen in lipodystrophy after six years of observation. We recognize that this patient may well have had a greater response than will be seen in the majority of patients. Indeed, his response is more impressive than that seen in many patients without HIV or lipodystrophy who begin a mild exercise programme. However, we present these data to alert clinicians and patients to the potential benefit of diet and exercise modification as treatment for lipodystrophy while our study and others continue. Although the effect of this regimen on abdominal obesity, insulin resistance and hyperlipidaemia was profound, no effect on peripheral fat atrophy was seen. Exercise and diet should be considered part of the treatment for lipodystrophy before “drug interruption”.
Further study of the optimal diet and exercise strategy for lipodystrophy is warranted.
REFERENCES
- 1.James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier; 2006. [Google Scholar]
- 2.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R, Schmitz H, Bairos L, Layne J, Potts E, Cloutier GJ, et al. Reduction of abdominal obesity in lipodystrophy associated with human immunodeficiency virus infection by means of diet and exercise: case report and proof of principle. Clin Infect Dis. 2002;34:390–393. doi: 10.1086/338402. [DOI] [PubMed] [Google Scholar]
- 4.Giralt M, Domingo P, Guallar JP, Rodriguez de la Concepcion ML, Alegre M, Dominique JC, et al. HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV-1/HAART-associated lipodystrophy. Antivir Ther. 2006;6:729–740. [PubMed] [Google Scholar]