Abstract
Aberrant angiogenesis is implicated in diseases affecting nearly 10% of the world’s population. The most widely used anti-angiogenic drug is bevacizumab, a humanized IgG1 monoclonal antibody that targets human VEGFA. Although bevacizumab does not recognize mouse Vegfa, it inhibits angiogenesis in mice. Here we show bevacizumab suppressed angiogenesis in three mouse models not via Vegfa blockade but rather Fc-mediated signaling through FcγRI (CD64) and c-Cbl, impairing macrophage migration. Other approved humanized or human IgG1 antibodies without mouse targets (adalimumab, alemtuzumab, ofatumumab, omalizumab, palivizumab and tocilizumab), mouse IgG2a, and overexpression of human IgG1-Fc or mouse IgG2a-Fc, also inhibited angiogenesis in wild-type and FcγR humanized mice. This anti-angiogenic effect was abolished by Fcgr1 ablation or knockdown, Fc cleavage, IgG-Fc inhibition, disruption of Fc-FcγR interaction, or elimination of FcRγ-initated signaling. Furthermore, bevacizumab’s Fc region potentiated its anti-angiogenic activity in humanized VEGFA mice. Finally, mice deficient in FcγRI exhibited increased developmental and pathological angiogenesis. These findings reveal an unexpected anti-angiogenic function for FcγRI and a potentially concerning off-target effect of hIgG1 therapies.
Introduction
Dozens of monoclonal antibodies are approved by the United States Food and Drug Administration, European Medicines Agency, and other regulatory agencies for treating numerous diseases including age-related macular degeneration (AMD), asthma, autoimmune disorders and multiple cancers. These drugs are used in millions of people worldwide with global sales exceeding $50 billion.1 In addition, there are hundreds of ongoing clinical trials evaluating various other monoclonal antibodies.1
Bevacizumab (Avastin), a humanized monoclonal IgG1 that targets VEGFA,2 inhibits blood vessel growth and has been approved for treating multiple cancers,3 and is widely used to treat neovascular AMD.4 Bevacizumab is exquisitely specific for human VEGFA, having no measurable binding affinity for, or ability to functionally inhibit, murine Vegfa.5–7 Surprisingly, numerous reports claim an anti-angiogenic effect of bevacizumab in various murine models of neovascularization.8–14 Yet nearly all these reports have compared bevacizumab with saline or no treatment controls rather than to a biologically appropriate human IgG1 control. We suspected, therefore, that the angioinhibitory effect of bevacizumab in murine models was misattributed to blockade of Vegfa, and was instead due to an intrinsic property of the IgG1 molecule independent of its antigenic specificity, namely a target-independent effect.
In this study, we found that bevacizumab, and numerous other therapeutic human IgG1 antibodies, as well as mouse IgG2a, suppressed angiogenesis in mice via FcγRI, the high-affinity IgG receptor.15–17 These effects were observed both with local and systemic administration of these antibody preparations at doses similar to or identical to those used in humans for various diseases.
A prospective randomized clinical trial reported in patients with corneal angiogenesis that bevacizumab, a full-length antibody that neutralizes human VEGFA activity and is able to bind FcγRs, is superior to ranibizumab, a humanized IgG1 Fab fragment that blocks human VEGFA but cannot bind FcγRs, in inhibiting angiogenesis.18 Our findings provide a molecular basis for this clinical observation. In contrast, clinical trials in patients with choroidal angiogenesis found no significant difference in the effects of bevacizumab versus ranibizumab, each tested at a single dose, on angiogenic lesion size.4,19 Our findings suggest that the dose of bevacizumab required to achieve FcγRI-mediated anti-angiogenic activity is roughly eight times higher than the dose used in these trials, which is sufficient only to neutralize human VEGFA, thereby providing a molecular rationale for testing such higher doses.
Angiogenic diseases collectively affect half-a-billion people;20 together, our data provide evidence that human IgG1 antibodies, as a class, form an important group of angioinhibitors, potentially fill the need for developing inexpensive generic human IgG1 drugs,21 and raise awareness for monitoring possible unintended effects on blood vessels by these widely used therapeutics. We also found increased pathological and developmental angiogenic responses in mice lacking FcγRI, suggesting that endogenous Igs also have a role in vascular patterning.
Materials and methods
Animals
All animal experiments were in accordance with the guidelines of the relevant institutional authorities. Male mice, aged 4–8 weeks, were randomized 1:1 to treatment with active drug versus inactive drug or control treatments.
Corneal angiogenesis
Nylon sutures (Mani, Utsunomiya, Japan) were placed into the corneal stroma of mice, and on day 10 after injury, we calculated the mean percentage CD31+Lyve1− blood vessel areas for corneal flat mounts with ImageJ (US National Institutes of Health, Bethesda, MD, USA) as previously reported.22,23
Choroidal angiogenesis
Laser photocoagulation (OcuLight GL, IRIDEX, Mountain View, CA, USA) was performed on both eyes of mice to induce neovascularization, and on day 7 after injury, choroidal angiogenesis volumes were measured by scanning laser confocal microscopy (TCS SP5, Leica, Wetzlar, Germany) as previously reported with 0.7% FITC-conjugated Isolectin B4 (Vector Laboratories, Burlingame, CA, USA).24,25 For intravitreous administration in choroidal angiogenesis experiments, drugs was administered into the vitreous humor of mice using a 33-gauge double-calibre needle (Ito Corporation, Tokyo, Japan) as previously described.26
Hind limb ischemia angiogenesis
Unilateral proximal femoral artery ligation was performed as previously described,27 and on day 7 after surgery, both anterior and posterior muscles from ischemic and non-ischemic hind limbs were collected and processed for immunohistochemical analysis for vessel quantification. Color laser Doppler analysis was also performed using a dedicated Laser Doppler Perfusion Imaging System (LDPI, PeriScan PIM II System, Perimed AB, Järfälla, Sweden).
Statistical analyses
Choroidal angiogenesis volumes per laser lesion were compared by hierarchical logistic regression using repeated measures analysis as previously described.28 For other comparisons, we used the Mann–Whitney U-test with Bonferroni correction for statistical comparison of multiple variables. Results are expressed as mean±s.e.m. Type-I error not exceeding 0.05 was deemed significant.
Results
Bevacizumab and human IgG1 antibodies inhibit angiogenesis in mice
Bevacizumab has no detectable binding to mouse Vegfa by surface plasmon resonance and does not block mouse Vegfa-induced retinal capillary endothelial cell proliferation.5–7 To further verify that bevacizumab does not functionally neutralize mouse Vegfa, we tested its ability to inhibit the activation of the Vegfr2 receptor tyrosine kinase in mouse Py4 hemangioma endothelial cells. As expected, bevacizumab inhibited Vegfr2 phosphorylation induced by human VEGFA but not by mouse Vegfa (Figure 1a).
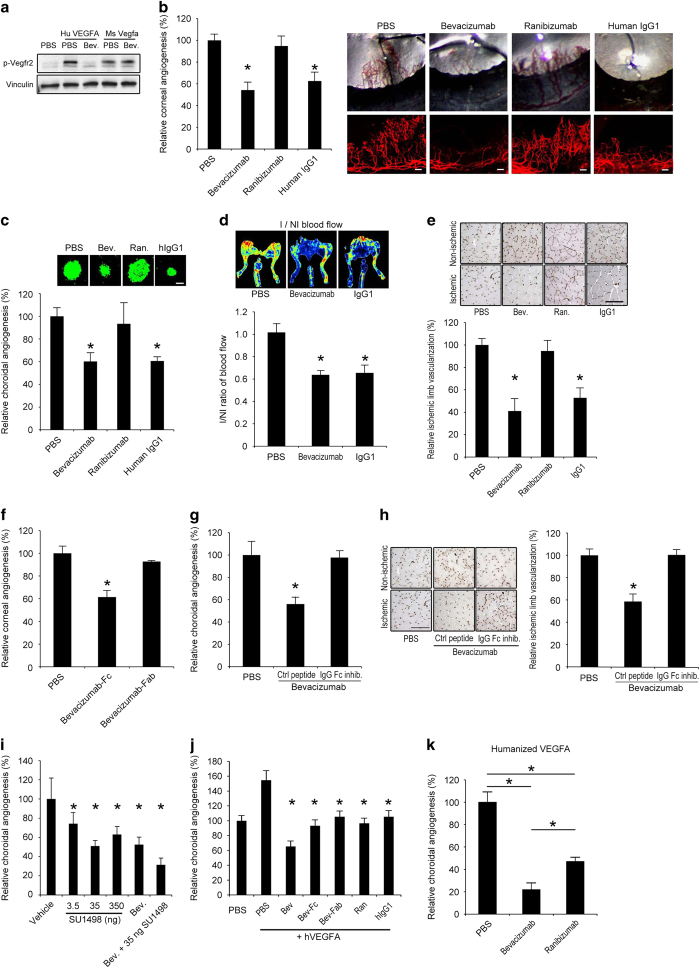
Figure 1.
Bevacizumab inhibited mouse angiogenesis via Fc region. (a) Western blot shows that bevacizumab inhibited Vegfr2 phosphorylation (pVegfr2) in Py4 mouse hemangioma endothelial cells when treated with human VEGFA, but not when treated with mouse Vegfa, after 10 min. Image representative of three experiments. (b) Bevacizumab and human IgG1, but not ranibizumab, decreased corneal angiogenesis in wild-type mice. Area of angiogenesis was measured 10 days after suture injury and normalized to PBS group. n=10–38. Representative photos of wild-type mouse eyes (upper row) and corneal flat mounts (lower row) showing reduced growth of blood vessels (CD31+, red) in eyes treated with bevacizumab or human IgG1, but not in eyes treated with ranibizumab. Scale bars, 100 μm. (c) Bevacizumab and human IgG1, but not ranibizumab, suppressed choroidal angiogenesis in wild-type mice 7 days after laser injury compared with PBS (experiment performed in JA laboratory). Images depict representative choroidal angiogenesis lesions (endothelial cells stained in green) in each treatment group. n=12–20. (d, e) Treatment of ischemic hind limb with bevacizumab or human IgG1 in wild-type mice suppressed muscle revascularization and decreased blood vessel perfusion, as seen in representative laser Doppler perfusion images (top), and measured blood flow in the ischemic limbs (bottom), normalized to the contralateral non-ischemic limbs, 7 days after surgery. n=6. I/NI, ischemic/non-ischemic. Bevacizumab and human IgG1, but not ranibizumab, treatment of ischemic limbs reduced muscle angiogenesis (CD31+, brown) as seen in representative images of muscle CD31 immunolocalization (e), and quantification of muscle CD31 immunolocalization (bottom), normalized to the contralateral non-ischemic limbs. (f) The Fc fragments, not the Fab fragment, of bevacizumab suppressed corneal angiogenesis in wild-type mice. Area of angiogenesis was measured 10 days after suture injury and normalized to PBS group. n=10–38. (g) Co-administration of a peptide that prevents IgG-Fc binding to FcγR, but not a control peptide, blocked inhibition of choroidal angiogenesis by bevacizumab in wild-type mice. (h) Co-administration of an IgG-Fc inhibitory peptide, but not a control peptide, blocked inhibition of muscle angiogenesis (CD31+, brown) by bevacizumab, as seen in representative images of muscle CD31 immunolocalization (left), and quantification of muscle CD31 immunolocalization (right), normalized to the contralateral non-ischemic limbs. Scale bar, 100 μm. n=6. (i) Bevacizumab suppressed choroidal angiogenesis in wild-type mice to the same extent as SU1498, a small molecule tyrosine kinase inhibitor of Vegfr2. Combined administration of bevacizumab and SU1498 suppressed choroidal angiogenesis to a greater extent than either of the agents alone. n=6. (j) Choroidal angiogenesis, augmented by administration of human VEGFA, was suppressed to similar extents by ranibizumab, bevacizumab-Fab, bevacizumab-Fc and human IgG1; and, to a greater extent, by bevacizumab. n=6–8. (k) Bevacizumab suppressed choroidal angiogenesis to a greater extent than ranibizumab in the humanized VEGFA mouse, a transgenic model that expresses a VEGFA protein that can be neutralized by both bevacizumab and ranibizumab. n=6. Results are means±s.e.m. *P<0.05 compared with PBS (b–h, k) or with vehicle (i) or with PBS+human VEGFA (j).
We tested the effects of bevacizumab in a mouse model of suture injury-induced corneal angiogenesis, which is pathophysiologically relevant to the human condition and is driven in large part by Vegfa. We found that administration of bevacizumab into the cornea by intrastromal injection (4 μl of the commercial 25 mg/ml preparation—a dose similar to that used in humans, when corrected for relative size) inhibited corneal angiogenesis by 46% compared with phosphate-buffered saline (PBS) administration in wild-type mice (Figure 1b). This angioinhibition occurred in a dose-dependent fashion (Supplementary Figure 1). We also tested whether ranibizumab, a humanized monoclonal IgG1 Fab fragment that binds human VEGFA but not mouse Vegfa6,29 and is approved for the treatment of neovascular AMD, was anti-angiogenic in this model. We confirmed that, like bevacizumab, ranibizumab inhibited Vegfr2 phosphorylation induced by human VEGFA but not by mouse Vegfa (Supplementary Figure 2). However, unlike bevacizumab, ranibizumab, at equimolar amounts, did not inhibit corneal angiogenesis (Figure 1b). Interestingly, the control isotype human IgG1 for bevacizumab also reduced corneal angiogenesis in wild-type mice in a dose-dependent fashion (Figure 1b and Supplementary Figure 3).
We next tested a mouse model of laser injury-induced choroidal angiogenesis, a widely used model of neovascular AMD that is driven in large part by Vegfa and has been predictive of the success of anti-VEGFA therapies in humans. Independent testing by three different laboratories (JA, YO and HT) determined that intraocular administration of bevacizumab (1 μl of the commercial 25 mg/ml preparation—a dose approximately eight times that was used in humans, when corrected for relative size) by intravitreous injection inhibited choroidal angiogenesis by 40–45% in wild-type mice compared with PBS administration, whereas an equimolar amount of ranibizumab did not do so (Figure 1c and Supplementary Figure 4). Similar to the corneal model, the isotype human IgG1 and human IgG1-Fc also suppressed choroidal angiogenesis in wild-type mice (Figure 1c and Supplementary Figure 4).
We tested the effect of bevacizumab in a third model of angiogenesis, induced by hind limb ischemia produced by femoral artery ligation, which is a model of peripheral arterial disease. Intramuscular injection of either bevacizumab or isotype control human IgG1 suppressed limb revascularization and diminished perfusion, as monitored by color laser Doppler imaging, compared with PBS injection (Figure 1d). There was a corresponding reduction in angiogenesis by 47–59% in the bevacizumab- or human IgG1-treated limbs compared with the PBS-treated limbs, whereas ranibizumab did not suppress angiogenesis (Figure 1e). These data support the concept that human IgG1 antibodies can suppress angiogenesis in multiple tissues.
Fc portion of human IgG1 critical for angioinhibition
Since bevacizumab and ranibizumab had nonsynonymous effects on angiogenesis in these mouse models, we suspected that the anti-angiogenic action of bevacizumab was due not to Vegfa neutralization but rather to IgG1-Fc-mediated effects. We confirmed that angioinhibition was due to the Fc, and not Fab, portion of bevacizumab by administering separately its Fab and Fc fragments as prepared from papain enzymatic digestion of the antibody (Supplementary Figure 5). Bevacizumab-Fab, but not bevacizumab-Fc, inhibited VEGFA-induced Vegfr2 phosphorylation, consistent with the location of the VEGFA-binding residues on the Fab fragment and indicating that the VEGFA neutralizing properties of bevacizumab were not affected by enzymatic digestion (Supplementary Figure 5). In contrast, bevacizumab-Fc, but not bevacizumab-Fab, reduced corneal angiogenesis in wild-type mice (Figure 1f), indicating that bevacizumab’s angioinhibitory activity in mice is due to its Fc domain and not because of binding Vegfa.
Human IgG1 and human IgG1-Fc also suppressed choroidal angiogenesis in wild-type mice (Figure 1c and Supplementary Figure 4). A peptide that prevents the binding of IgG to FcγRs by interacting with the Fc portion of IgG (IgG-Fc peptide inhibitor),30 but not a control peptide, eliminated the ability of bevacizumab to inhibit choroidal and hind limb angiogenesis in wild-type mice (Figure 1g and h), confirming a role for FcγR in these models.
We sought to determine whether human IgG1s would suppress angiogenesis not only when exogenously administered but also if produced endogenously. Therefore, we performed in vivo transfection of a plasmid encoding human IgG1-Fc coupled to an IL2-secretory sequence (phIgG1-Fc) by injecting it into the subretinal space of wild-type mice before laser injury. We found that phIgG1-Fc reduced choroidal angiogenesis in wild-type mice compared with a control null plasmid (Supplementary Figure 6). These data show that angiogenesis can be suppressed not only by exogenous administration of human IgG1 antibodies but also by endogenous overexpression of their Fc region.
Bevacizumab and human IgG1 antibodies inhibit angiogenesis in humanized VEGF mice
Ranibizumab, which targets human VEGFA and does not have an Fc region, is effective in treating neovascular AMD in humans.31,32 We sought to compare the relative anti-angiogenic efficacies of bona fide VEGFA targeting and of FcγR-mediated signaling. First we found that the extent of suppression of choroidal angiogenesis by bevacizumab (48%) in wild-type mice was similar to that achieved by SU1498 (49%), a small molecule tyrosine kinase inhibitor of Vegfr2 (Figure 1i), and to that achieved by various inhibitors of Vegfa reported in previous studies (~40–55%).28,33–36 We also found that bevacizumab potentiated the angioinhibitory effects of SU1498 (Figure 1i).
Next we used a model in which laser injury-induced choroidal angiogenesis is augmented by prior intravitreous administration of human VEGFA.37 In this model where angiogenesis is driven both by human VEGFA and endogenous mouse pathways, we found that ranibizumab and bevacizumab-Fab, which target human VEGFA but do not contain an Fc region, suppressed angiogenesis to a similar extent as bevacizumab-Fc and human IgG1, which contain an Fc region but do not target human VEGFA (Figure 1j). In addition, full-length bevacizumab suppressed angiogenesis to a greater extent than any of the pure anti-VEGFA or Fc-containing agents alone, further indicating that modulating these two anti-angiogenic pathways simultaneously can be additive. Next, we studied ‘humanized VEGFA’ mice,6 wherein the mouse Vegfa gene was mutated such that its protein product could be neutralized by bevacizumab and ranibizumab. In this model, we found that bevacizumab suppressed choroidal angiogenesis to a significantly greater extent than ranibizumab (Figure 1k). Collectively these data demonstrate that the Fc region of bevacizumab can potentiate its anti-angiogenic effect in systems where human VEGFA is present.
FcγRI necessary for human IgG1-induced angioinhibition
We performed additional experiments to investigate the nature of the Fc-mediated anti-angiogenic effect of bevacizumab. It is known that deglycosylation of human IgG1 dramatically reduces its binding to both human and mouse FcγRs.38–40 We found that deglycosylated bevacizumab, despite retaining its ability to inhibit human VEGFA-induced Vegfr2 phosphorylation (Supplementary Figure 7), did not reduce choroidal angiogenesis in wild-type mice (Figure 2a). These data suggest that the anti-angiogenic effect of bevacizumab in mice is mediated by an endogenous FcγR that binds human IgG1.40
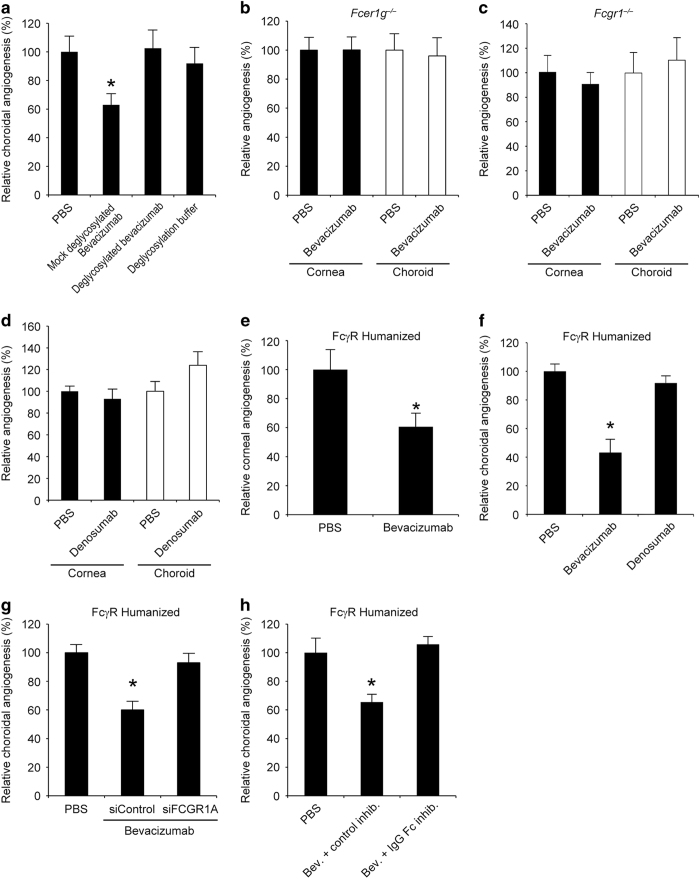
Figure 2.
Bevacizumab inhibited mouse angiogenesis via FcγRI. (a) Deglycosylated bevacizumab did not suppress choroidal angiogenesis in wild-type mice; however, choroidal angiogenesis was inhibited by bevacizumab subjected to mock treatment. The deglycosylation buffer had no effect on choroidal angiogenesis. n=8–14. (b) Bevacizumab did not suppress corneal or choroidal angiogenesis in Fcer1g−/− mice. n=8–10. (c) Bevacizumab did not inhibit corneal or choroidal angiogenesis in Fcgr1−/− mice. No significant difference between groups. n=10–13. (d) Denosumab did not suppress corneal or choroidal angiogenesis in wild-type mice. n=6–8. No significant difference between groups. (e) Bevacizumab inhibited corneal angiogenesis in FcγR humanized mice. n=8. Results are means±s.e.m. *P<0.05 compared with PBS. (f) Bevacizumab, but not denosumab, inhibited choroidal angiogenesis in FcγR humanized mice. n=6–8. (g) Co-administration of a 17+2-nt cholesterol conjugated human FCGR1A siRNA, but not a 17+2-nt cholesterol-conjugated control Luc siRNA, blocked inhibition of choroidal angiogenesis by bevacizumab in FcγR humanized mice. n=8. (h) Co-administration of an IgG-Fc inhibitory peptide, but not a control peptide, blocked inhibition of choroidal angiogenesis by bevacizumab in FcγR humanized mice. n=8. Results are means±s.e.m. *P<0.05 compared with PBS (a, e–h).
We found that bevacizumab did not suppress choroidal angiogenesis in Fcer1g−/− (a.k.a. FcRγ−/−) mice (Figure 2b), which lack the common gamma chain of the activating FcγRs: FcγRI, FcγRIII and FcγRIV. To determine which activating FcγR was responsible, we tested mice lacking these receptors. First, we tested the involvement of FcγRI (encoded by Fcgr1), and found that bevacizumab failed to inhibit corneal or choroidal angiogenesis in Fcgr1−/− mice (Figure 2c). In contrast, bevacizumab inhibited corneal and choroidal angiogenesis in mice lacking Fcgr3 (Supplementary Figure 8), which encodes FcγRIII, and in mice lacking Fcgr4 (Supplementary Figure 9), which encodes FcγRIV. Supporting the latter result, bevacizumab did not inhibit angiogenesis in Fcgr1−/−; Fcgr2b−/−; Fcgr3−/−; Fcer1a−/− and Fcer2a−/− mice, which express FcγRIV but not any of the other IgG or IgE receptors41 (Supplementary Figure 10). Human IgG2 binds to mouse FcγRII and FcγRIII, but not to FcγRI.40 The human IgG2 denosumab (Prolia: anti-RANKL) did not inhibit corneal or choroidal angiogenesis in wild-type mice (Figure 2d), suggesting that binding to FcγRI is required for IgG-induced angioinhibition.
We also found that subretinal transfection of a plasmid encoding a mutant form of human IgG1-Fc engineered with point mutations that eliminate binding to FcγRI or of a plasmid encoding human IgG2-Fc did not suppress choroidal angiogenesis in wild-type mice (Supplementary Figure 11), further supporting the concept that angioinhibition is a target-independent class effect of human or humanized IgG1 monoclonal antibodies that is mediated via FcγRI.
Administration of bevacizumab by i.v. injection every other day (to account for the 6.5-fold higher serum clearance rate in mice compared with humans,42 in whom it is administered weekly or every other week) also suppressed choroidal angiogenesis in wild-type mice in a dose-dependent fashion, but did not do so in Fcgr1−/− mice (Supplementary Figure 12). Collectively, these data indicate that bevacizumab reduces mouse angiogenesis via FcγRI and not via Vegfa inhibition.
Bevacizumab reduces angiogenesis in FcγR humanized mice
Although human IgG1 binds both mouse FcγRI and human FcγRI,40 the structural diversity and unique cellular expression patterns of mouse and human FcγRs are not synonymous.43 The generation of an FcγR humanized mouse via transgenic expression of the entire human FcγR family, under the control of their human regulatory elements, on a genetic background lacking all mouse FcγRs has enabled better prediction of the functional consequences of engaging human FcγRs by IgGs.44 In these FcγR humanized mice, we found that intracorneal bevacizumab reduced corneal angiogenesis just as in wild-type mice (Figure 2e). Bevacizumab also reduced choroidal angiogenesis in FcγR humanized mice whereas the human IgG2 denosumab did not (Figure 2f). As human IgG2 can bind human FcγRII and human FcγRIII but not human FcγRI,45 this result supports the notion that interaction with human FcγRI is mandatory for the anti-angiogenic effect of IgGs in our models. The angioinhibitory effect of bevacizumab was blocked by both the IgG-Fc peptide inhibitor and a cholesterol-conjugated28 FCGR1A siRNA (Figure 2g and h). These data demonstrate that target-independent FcγRI-mediated angioinhibitory activity of humanized monoclonal IgG1 antibodies is operative in a FcγR humanized system.
Bevacizumab interacts with FcγRI and initiates signaling in vivo
As we found that bevacizumab suppressed angiogenesis via FcγRI, we tested whether bevacizumab binds FcγRI in vivo using two complementary strategies. First, using a pull-down assay, we found that biotinylated bevacizumab, but not denosumab, that was injected into wild-type mouse corneas following suture injury co-precipitated with mouse FcγRI (Figure 3a). Next, we injected unlabeled bevacizumab into the corneas of FcγR humanized mice that were subjected to suture injury, and found that immunoprecipitation of human FcγRI pulled down human IgG1 (Figure 3b). Collectively, these data demonstrate an in vivo interaction between bevacizumab and both human and mouse FcγRI. In addition, bevacizumab injected into the corneas of FcγR humanized mice following suture injury-induced FcγRI phosphorylation (Figure 3b).
Figure 3.
Bevacizumab interacted with, induced phosphorylation of, and upregulated abundance of FcγRI in vivo. (a) Wild-type mouse corneas that had been administered biotinylated bevacizumab or biotinylated denosumab following suture injury were subjected to streptavidin pull-down and immunoblotting for mouse FcγRI. Biotinylated bevacizumab, but not denosumab, interacted with mouse FcγRI in vivo. Anti-streptavidin immunoblotting confirmed efficient pull-down of both biotinylated antibodies. (b) FcγR humanized mouse corneas that had been administered bevacizumab or PBS following suture injury were subjected to immunoprecipitation of human FcγRI followed by immunoblotting for human IgG1 or phosphotyrosine. Bevacizumab, but not PBS, interacted with and induced phosphorylation of human FcγRI in vivo. Reprobing confirmed efficient immunoprecipitation of human FcγRI in both bevacizumab- and PBS-treated corneas. Each image is representative of three experiments (a, b). (c) Bevacizumab, but not PBS, increased Fcgr1 mRNA abundance in RAW264.7 mouse macrophages and in wild-type mouse corneas following suture injury, as monitored by real-time reverse transcription PCR, and FcγRI protein abundance in RAW264.7 cells, as monitored by western blotting. Densitometry of FcγRI normalized to Vinculin shown. n=4–6. Results are means±s.e.m. *P<0.05 compared with PBS.
Crosslinking of FcγRI by human IgG1 aggregates can activate FcγRI. However, we found, using dynamic light scattering, no evidence of aggregation of bevacizumab at the administered dose (Supplementary Figure 13), as might be expected from a clinical grade preparation. This suggests that monomeric bevacizumab can induce FcγRI-mediated signaling in vivo in the systems we studied. Indeed, monomeric IgG engagement of other activating FcγRs has been shown to induce phosphorylation and signaling.46,47 Nevertheless, we cannot exclude the possibility that once the non-aggregated liquid formulation is administered into the mouse, bevacizumab might undergo in vivo aggregation. However, this seems unlikely given the lack of any known mouse ligand for bevacizumab. Moreover, if such in vivo aggregation of bevacizumab occurred in the mouse, it would also be expected to occur in human eyes because of the similar dose injected and the presence of a bona fide ligand—human VEGFA.
We next wondered how bevacizumab could bind FcγRI given the high serum concentration of endogenous mouse IgG that might be expected to compete for binding. Indeed, it has been shown that FcγR can still bind IgG under serum conditions48,49 and can execute numerous biological functions in vivo in response to exogenous mouse IgG2a and human IgG1 antibodies.50–58 This ability of FcγRI to contribute to biological signaling has been attributed to the short half-life of the interaction of FcγRI with its ligand (turnover within minutes), de novo synthesis of free FcγRI, receptor reorganization or conformational changes on the membrane, sampling IgG as a scavenger receptor, and ‘inside-out’ stimulation by cytokines that rapidly increases the binding of FcγRI to exogenous monomeric IgG.48,49,51,56,59,60 Indeed we found that bevacizumab increased FcγRI levels in mouse macrophages and in wild-type mouse corneas following suture injury (Figure 3c).
More importantly, we found that the concentrations of endogenous mouse IgG2c (an allelic variant of mouse IgG2a that is expressed in C57BL/6 mice61,62) in the extravascular portion of the injured tissues are minute compared with circulating levels and far lower than the extravascular tissue concentrations of exogenously administered bevacizumab (Supplementary Figure 14a and b). This paucity of extravascular mouse IgG (Supplementary Figure 14c) and the excess of bevacizumab in the injured tissues combined with increased FcγRI abundance can explain the ability of the exogenous human IgG1 to bind FcγRI in vivo on extravascular cells, e.g., macrophages, which express FcγRI63 and can modulate angiogenesis,64 and initiate signaling.
Numerous therapeutic human IgG1s inhibit angiogenesis via FcγRI
Next we assessed the anti-angiogenic effects of several human or humanized IgG1 monoclonal antibodies that are approved for treatment of various human diseases, and either do not bind the mouse homologs of their intended human protein targets or have no mammalian target: adalimumab (Humira: anti-TNFα), alemtuzumab (Campath: anti-CD52), ofatumumab (Arzerra: anti-CD20), omalizumab (Xolair: anti-IgE), palivizumab (Synagis: anti-respiratory syncytial virus protein F), and tocilizumab (Actemra: anti-IL-6R). All of these human IgG1 antibodies reduced both corneal and choroidal angiogenesis in wild-type mice (Figure 4a and b) in contrast to the human IgG2 denosumab (Figure 2e). We tested two of these antibodies—omalizumab and palivizumab—in Fcgr1−/− mice, and found that they did not suppress corneal or choroidal angiogenesis (Figure 4c). We also found that a mutant version of alemtuzumab (G1Δab), which was engineered with point mutations in the CH2 domain of its Fc region that eliminate binding to FcγRI and reduce binding to other FcγRs,65 yet retains binding to human CD52 (Supplementary Figure 15a and b), did not inhibit corneal or choroidal angiogenesis in wild-type mice (Supplementary Figure 15d and e). Conversely, we found that another mutant version of alemtuzumab (D270A), which preserves binding to FcγRI but not to FcγRII and FcγRIII,66,67 suppressed choroidal angiogenesis in wild-type mice but not in Fcgr1−/− mice (Supplementary Figure 15f).
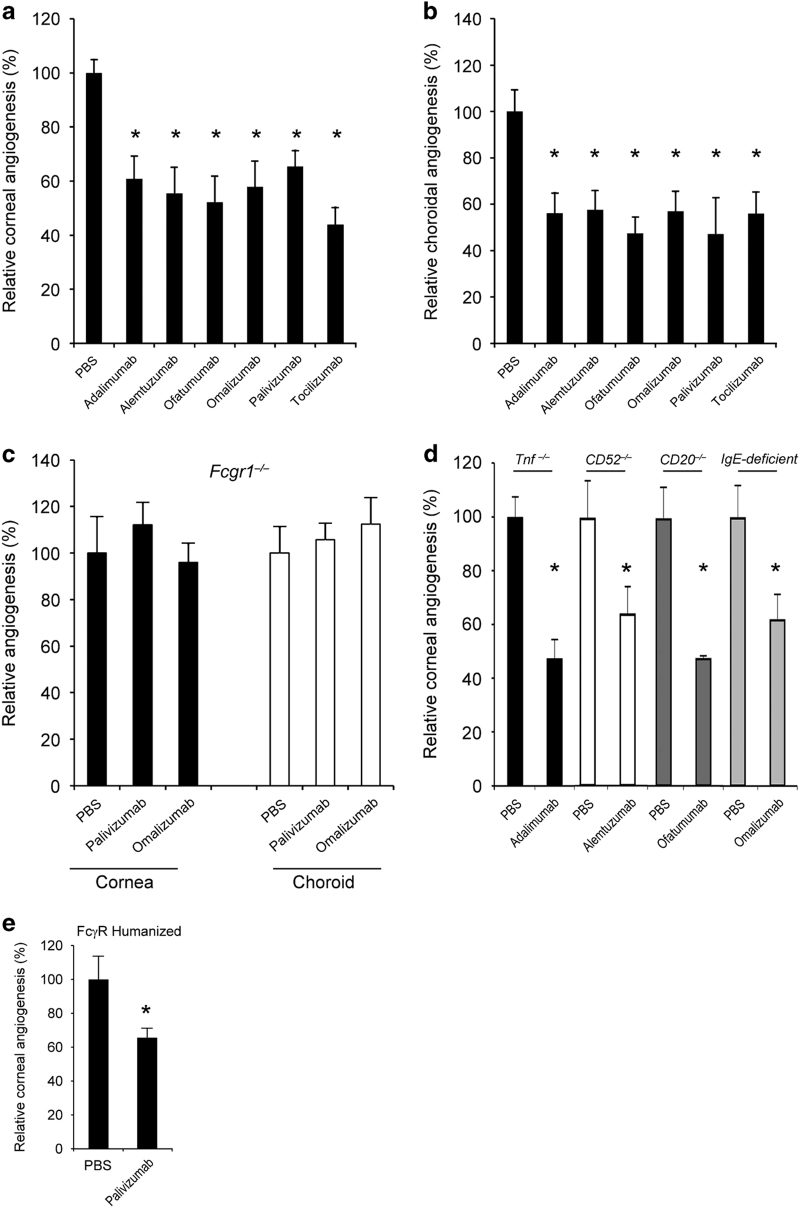
Figure 4.
Human IgG1s inhibited mouse angiogenesis via FcγRI. Treatment with the human IgG1 antibodies adalimumab, alemtuzumab, ofatumumab, omalizumab, palivizumab or tocilizumab reduced (a) corneal and (b) choroidal angiogenesis in wild-type mice. n=8–19. (c) Palivizumab and Omalizumab did not inhibit corneal or choroidal angiogenesis in Fcgr1−/− mice. n=6–8. No significant difference between groups. (d) Adalimumab, a human anti-TNFα monoclonal antibody, inhibited corneal angiogenesis in Tnf−/− mice. n=9. Alemtuzumab, a humanized anti-CD52 monoclonal antibody, inhibited corneal angiogenesis in CD52−/− mice. n=8. Ofatumumab, a human anti-CD20 monoclonal antibody, inhibited corneal angiogenesis in CD20−/− mice. n=8. Omalizumab, a humanized anti-IgE monoclonal antibody, inhibited corneal angiogenesis in IgE-deficient mice. n=10. (e) Palivizumab inhibited choroidal angiogenesis in FcγR humanized mice. Results are means±s.e.m. *P<0.05 compared with PBS (a, b, d, e).
We sought to exclude the possibility that the observed angioinhibition could be due to unforeseen or illegitimate interaction between these human antibodies and the mouse homologs of their human protein targets by testing them in mice deficient for the homologous genes. Such interactions were not responsible for the angiosuppression as we found that corneal angiogenesis was inhibited by adalimumab in Tnf−/− mice, alemtuzumab in CD52−/− mice, ofatumumab in CD20−/− mice and omalizumab in IgE-deficient mice (Figure 4d), just as in wild-type mice. Collectively, these data indicate that multiple therapeutic human IgG1 antibodies can suppress angiogenesis via FcγRI and independent of their intended target.
Next we tested some of these antibodies in FcγR humanized mice. We found that intracorneal palivizumab reduced corneal angiogenesis (Figure 4e). In addition, alemtuzumab, but not alemtuzumab G1Δab, which does not bind FcγRI, suppressed choroidal angiogenesis in FcγR humanized mice (Supplementary Figure 16). These data demonstrate that target-independent angioinhibitory activity of humanized monoclonal IgG1 antibodies is operative in a FcγR humanized system.
Mouse IgG2a and mouse IgG2c inhibit angiogenesis via FcγRI
To determine whether antibodies potentially produced by mice against human IgGs might have a role in the angioinhibition we observed, we tested Rag2−/− mice, which lack B and T cells and are devoid of Igs. Bevacizumab inhibited corneal and choroidal angiogenesis in Rag2−/− mice (Supplementary Figure 17), indicating that such an immune response potentially mounted against bevacizumab is not responsible for its angioinhibitory effect.
To exclude other potential cross-species biological effects, we tested mouse IgG2a, which like human IgG1 binds to FcγRI with high affinity.17,41,57,68,69 Intracorneal or subretinal transfection of a plasmid encoding mouse IgG2a-Fc coupled to an IL2-secretory sequence inhibited corneal or choroidal angiogenesis, respectively, in wild-type mice (Supplementary Figure 18). In contrast, a plasmid encoding a mutant form of mouse IgG2a-Fc engineered with point mutations that eliminate binding to FcγRI and coupled to the same IL2-secretory sequence, did not suppress angiogenesis (Supplementary Figure 18). Recombinant mouse IgG2a-Fc inhibited choroidal angiogenesis in wild-type mice in a dose-dependent fashion, whereas mouse IgG2b-Fc, which has high binding affinity for FcγRIV but not for FcγRI,17,41,43,68,69 did not suppress angiogenesis (Supplementary Figure 19). In addition, neither recombinant mouse IgG2a-Fc nor a plasmid encoding mouse IgG2a-Fc reduced angiogenesis in Fcgr1−/− mice (Supplementary Figure 20). Further, mouse IgG2c also suppressed choroidal angiogenesis in wild-type mice but not in Fcgr1−/− mice (Supplementary Figure 21). Together these data further support the concept that suppression of angiogenesis via FcγRI is not limited to human IgG1 but also is a property of mouse IgG2a and mouse IgG2c.
Host Igs modulate angiogenesis
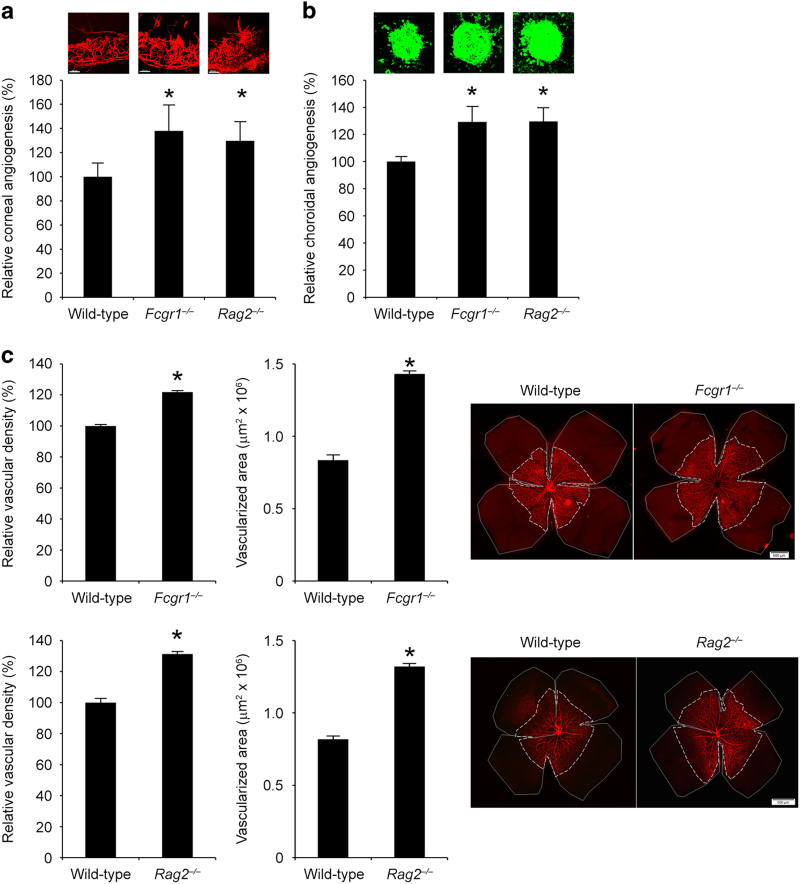
As we found that recombinant and endogenously over-expressed mouse IgG2a and mouse IgG2c suppressed injury-induced angiogenesis, we explored whether native host Igs modulate vascularization. Indeed, we found that corneal and choroidal angiogenesis responses to suture or laser injury (without administration of bevacizumab), respectively, were higher in Fcgr1−/− and Rag2−/− mice compared with littermate wild-type controls (Figure 5a and b). Physiological vascularization of the retina during development proceeds from the central optic nerve to the periphery. This process is not complete in mice until several days after birth. We found that at postnatal day 4, both the area of vascularized retina and density of retinal vessels were greater in Fcgr1−/− and Rag2−/− mice compared with littermate wild-type controls (Figure 5c). Taken together, these data suggest an anti-angiogenic role for endogenous Igs in vascular patterning both during development and response to injury that is mediated via FcγRI.
Figure 5.
Endogenous Igs suppressed mouse angiogenesis. Corneal angiogenesis area (a) and choroidal angiogenesis volume (b) are greater in Fcgr1−/− and Rag2−/− mice compared with wild-type mice. n=8–20. (c) The vascular density and total area of vascularized retina at postnatal day 4 is greater in Fcgr1−/− and Rag2−/− mice compared with wild-type mice. n=8. Results are means±s.e.m. *P<0.05 compared to wild-type mice (a–c). Vascular density in the retina is normalized to wild-type mice. Representative flat mounts of corneal (a, red), choroidal (b, green) and retinal (c, red), vessels are shown.
Human IgG1 reduces angiogenesis via bone marrow-derived cells expressing FcγRI
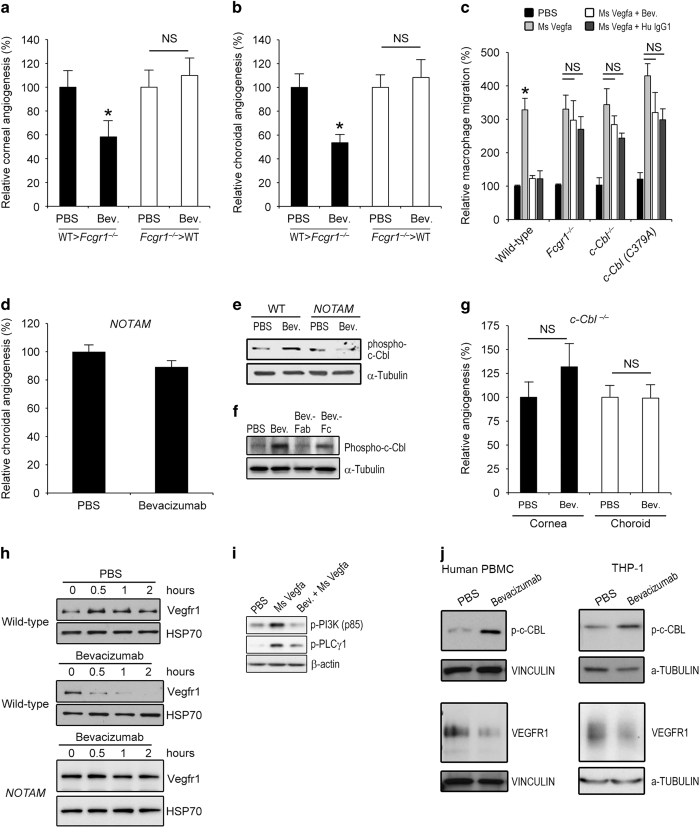
To determine whether bone marrow-derived or resident cell expression of FcγRI was the critical effector in IgG1 mAb-mediated angioinhibition, we created bone marrow chimeric mice. Bevacizumab suppressed corneal and choroidal angiogenesis in Fcgr1−/− mice receiving wild-type bone marrow but did not do so in wild-type mice receiving Fcgr1−/− bone marrow (Figure 6a and b). These results suggest that FcγRI in bone marrow-derived cells is critical for bevacizumab-induced angioinhibition.
Figure 6.
Bevacizumab inhibited angiogenesis via macrophage FcγRI and c-Cbl. Bevacizumab suppressed corneal (a) and choroidal (b) angiogenesis in Fcgr1−/− mice transplanted with wild-type mouse bone marrow, but not in wild-type mice receiving Fcgr1−/− bone marrow. n=11–16. (c) Bevacizumab and human IgG1 inhibited mouse Vegfa-induced migration, over 12 h, of bone marrow-derived macrophages isolated from wild-type mice but not from Fcgr1−/−, c-Cbl−/− or c-Cbl (C379A) mice, which lack E3 ubiquitin ligase activity. n=3. Results are means±s.e.m. *P<0.05 compared with PBS (a–c). (d) Bevacizumab did not inhibit choroidal angiogenesis in NOTAM mice. n=10. (e) Western blot shows induction of c-Cbl phosphorylation in wild-type mouse BMDMs treated with bevacizumab for 15 min. Protein loading was assessed by α-Tubulin abundance. (f) Western blot shows in vivo induction of c-Cbl phosphorylation in wild-type mouse corneas following suture injury that were treated with bevacizumab or its Fc fragment, but not by its Fab fragment. (g) Bevacizumab did not inhibit corneal or choroidal angiogenesis in c-Cbl−/− mice. n=11–30. NS, no significant difference between groups. (h) Western blots show time-dependent Vegfr1 degradation in wild-type but not NOTAM mouse BMDMs treated with bevacizumab. Protein loading was assessed by HSP70 abundance. (i) Western blots show that RAW264.7 mouse macrophages pre-treated with bevacizumab, but not PBS, 2 h before stimulation with mouse Vegfa, exhibited reduced phosphorylation of PI3K and PLCγ1 at 10 min after Vegfa exposure. Protein loading was assessed by β-actin abundance. (j) Western blots show induction of c-Cbl phosphorylation in human peripheral blood mononuclear cells (PBMC) or in THP-1 human monocytic cells, treated with bevacizumab, but not PBS, for 15 min. Treatment with bevacizumab, but not PBS, reduced VEGFR1 abundance in human PBMCs and THP-1 cells. Protein loading was assessed by Vinculin or α-Tubulin abundance. Images representative of three experiments (e, f, h–j).
Among the various types of bone marrow-derived cells, macrophages are best known to have a critical role in angiogenesis.70 Both bevacizumab and human IgG1 inhibited mouse Vegfa-induced migration of wild-type mouse bone marrow-derived macrophages (BMDMs) but not of Fcgr1−/− BMDMs (Figure 6c). Corroborating these data, we found that bevacizumab reduced the infiltration of F4/80+ macrophages into the sutured cornea, laser-injured choroid, and ischemic hind limb of wild-type mice (Supplementary Figure 22). These findings are in concert with the abundant expression of FcγRI by macrophages.63,71
We next assessed whether bevacizumab induces intracellular FcγR-mediated signaling events. First we tested the FcRγ-chain signaling-deficient NOTAM mice, which exhibit normal cell surface Fc receptor expression and normal IgG binding, but have non-signaling Fc receptors because their associated γ-chains have been mutated in their immunoreceptor tyrosine-based activation motif, which is responsible for signal transduction.72 We found that bevacizumab did not suppress choroidal angiogenesis in FcR NOTAM mice (Figure 6d), suggesting that this angioinhibition is dependent on FcγR-mediated signaling. Bevacizumab induced phosphorylation of FcγRI in the mouse cornea (Figure 3b); therefore, we examined the potential involvement of c-Cbl, a major regulator of tyrosine kinase signaling that is downstream of FcγRI-initiated signaling.73,74 We found that bevacizumab induced phosphorylation of c-Cbl in wild-type but not FcR NOTAM mouse BMDMs (Figure 6e). This was also corroborated in vivo: increased phosphorylation of c-Cbl was observed in the corneas of wild-type mice treated with bevacizumab and bevacizumab-Fc, but not bevacizumab-Fab, following suture injury (Figure 6f). Neither bevacizumab nor human IgG1 inhibited mouse Vegfa-induced migration of c-Cbl−/− BMDMs (Figure 6c). Further, we found that bevacizumab did not inhibit corneal or choroidal angiogenesis in c-Cbl−/− mice (Figure 6g), indicating that c-Cbl activation is essential for this process.
One of the principal signaling pathways employed by mouse Vegfa to induce macrophage migration is activation of Vegfr1 receptor tyrosine kinase and downstream activation of PI3K and PLCγ1.75–78 Via its E3 ubiquitin ligase activity, c-Cbl is capable of inducing degradation of numerous tyrosine kinases including Vegfr1.79 Indeed, we found that bevacizumab treatment of mouse macrophages induced degradation of Vegfr1 in wild-type but not FcR NOTAM BMDMs (Figure 6h). Bevacizumab also reduced mouse Vegfa-induced phosphorylation of PI3K and PLCγ1 in mouse macrophages (Figure 6i). Consistent with these findings, neither bevacizumab nor human IgG1 inhibited mouse Vegfa-induced migration of BMDMs isolated from c-Cbl (C379A) mutant mice (Figure 6c), which lack a functional RING finger domain necessary for the E3 ubiquitin ligase activity of c-Cbl.80 Also consistent with these findings, and the lack of angioinhibition observed in c-Cbl−/− mice, was the finding that bevacizumab did not reduce corneal angiogenesis in c-Cbl (C379A) mutant mice (Supplementary Figure 23). We also found that bevacizumab induced phosphorylation of c-CBL and degradation of VEGFR1 in primary human peripheral blood monocytes as well as in THP-1 human monocytes (Figure 6j).
Human IgG1 does not inhibit angiogenesis via ADCC, ADCP or CDC
Antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) are two-step processes initiated by full-length IgGs that couple Fab binding to a target cell antigen with Fc binding to an activating FcγR on an effector cell.71 These effector functions, as well as complement-dependent cytotoxicity (CDC) have a major role in the mode of action of several monoclonal antibodies employed in cancer therapy.81–83 Our findings that numerous human IgG1 antibodies, each with different Fab targeting domains (and none of which target mouse antigens), similarly suppressed angiogenesis argue against ADCC and ADCP as the mediators of this class effect. Moreover, bevacizumab-Fc and human IgG1-Fc, each devoid of Fab domains, also suppressed angiogenesis like full-length antibodies. We have already shown that bevacizumab inhibited corneal and choroidal angiogenesis in mice lacking FcγRIII, a receptor on NK cells that mediates ADCC,84 and in mice lacking FcγRIV, which also has an important role in ADCC85 (Supplementary Figures 8 and 9). In addition, bevacizumab inhibited corneal and choroidal angiogenesis in Il2rg−/− mice, which are deficient in NK cells (Supplementary Figure 24). These data support the thesis that this effector function is not involved in the angioinhibitory effect of bevacizumab in mice.
The inability of denosumab to suppress corneal or choroidal angiogenesis suggests that ADCC and CDC, which can be induced by both human IgG1 and human IgG2,86 are not responsible for angioinhibition induced by human IgG1s. We also found that a mutant version of alemtuzumab (G1Δa), which was engineered with point mutations in the CH2 domain of its Fc region that eliminate its CDC activity, yet retains binding to human CD52 and to FcγRI (Supplementary Figure 15a–c), inhibited corneal and choroidal angiogenesis in wild-type mice (Supplementary Figure 15d and e). In addition, bevacizumab suppressed choroidal angiogenesis in C1qa−/− mice (Supplementary Figure 25), which are deficient in complement C1QA, confirming that the angioinhibitory activity of human IgG1 antibodies does not require CDC. Subretinal transfection of a plasmid encoding a mutant form of human IgG1-Fc engineered with the K322A or D270A point mutations, which eliminates binding to C1q and induction of CDC while preserving binding to FcγRI,66,67,87 also reduced choroidal angiogenesis in wild-type mice (Supplementary Figure 26). Collectively, these data support the concept that angioinhibition is a target-independent class effect of human or humanized IgG1 monoclonal antibodies that is mediated via FcγRI, and not ADCC, ADCP or CDC.
Discussion
We have shown that human or humanized IgG1 antibodies are, as a class, angioinhibitory in multiple mouse models of ocular and muscle angiogenesis via Fc-dependent signaling. Our findings introduce angiosuppression to the list of important biological functions that are triggered by FcγRI in vivo.57 Exploiting this intrinsic property of human IgG1s could offer new therapeutic opportunities to treat diseases driven by angiogenesis that collectively affect nearly 10% of the world’s population.20 For example, several human IgG1 drugs or human IgG1-Fc fusion proteins approved for other indications could be repurposed as angiogenesis inhibitors. IVIg or non-targeted, ‘generic’ human IgG-Fc might represent even more inexpensive alternatives, as we demonstrate in a companion manuscript.88 Additional anti-angiogenic efforts might be directed toward developing peptides or small molecules that induce signaling via FcγRI or c-Cbl.
The dose of bevacizumab (100 μg) we injected into the mouse cornea, whose volume is ~2 μl, is similar in concentration to the dose of bevacizumab (2.5–5 mg) that has been administered into human corneas, whose volume is ~70 μl. Our findings suggest that in human corneas, bevacizumab would, at this dose, exert anti-angiogenic activity both via VEGFA inhibition and via FcγRI-mediated pathways, and that it might be expected to suppress angiogenesis to a greater extent than ranibizumab, which possesses only the anti-VEGFA activity. Indeed, a recent prospective randomized study reported that in humans with corneal angiogenesis, bevacizumab was superior to ranibizumab.18
In contrast, no significant difference was found between bevacizumab and ranibizumab in human eyes with choroidal angiogenesis due to neovascular AMD.4,19 We suggest that the reason for this lack of difference is that the amount of bevacizumab that is currently administered in these patients, while sufficient to neutralize VEGFA, is insufficient to induce FcγRI-mediated signaling. The dose of intravitreously administered bevacizumab required to suppress choroidal angiogenesis via FcγRI in mice (25 μg) translates, based on relative vitreous humor volumes, to ~10 mg in the human eye, which is eightfold the currently administered clinical dose. These values are compatible with the relative lower affinity of human IgG1-Fc for human FcγRI (K D=15–40 nmol/l)45,56,89 compared with that of bevacizumab for human VEGFA (K D=0.5–2.2 nmol/l).3,5 Our findings predict that a eightfold higher dose bevacizumab would achieve both VEGFA inhibition and FcγRI-mediated angioinhibition, and provide a rationale for testing such higher doses of bevacizumab or combining human IgG1-Fc to anti-human VEGFA drugs in patients with neovascular AMD to potentiate therapeutic angioinhibition.
It is reasonable to query whether it would be possible to inject 10 mg of bevacizumab into the human eye. The viability of injecting 10 mg of a biological drug has been demonstrated in a Phase 2 trial of lampalizumab, a Fab fragment. At present in the clinic, 1.25 mg of bevacizumab is injected in a 50-μl volume. Retina specialists routinely inject 100 μl of corticosteroids or 200 μl of antibiotics into the vitreous humor of humans for various disorders. With such higher delivery volumes, 10 mg of bevacizumab can be administered by increasing the concentration of the formulation from the current 25 mg/ml to 50–100 mg/ml, a value similar to that of therapeutic human IVIg preparations in current use. Alternatively, bevacizumab-Fc or human IgG1-Fc could be administered, at correspondingly lower doses, to induce FcγRI-dependent angioinhibition.
It would be interesting to explore to what extent the therapeutic effects of IgG1 antibodies used in the treatment of AMD, arthritis, asthma and solid tumors—disorders in which angiogenesis plays a critical role20—might be mediated by FcγRI. Our data also suggest that it might be prudent to monitor potential effects of human IgG1 antibodies on the vasculature in other diseases, as we demonstrate is the case in IVIg-treated patients in a companion manuscript.88 Indeed, the minimal angioinhibitory dose of bevacizumab in mice, 15 mg/kg, is used in humans with many forms of cancer, suggesting that at this dose in people, the drug might have dual anti-angiogenic activity: via VEGFA inhibition and FcγRI-dependent pathways. Although most human IgG1 antibodies are administered systemically at doses of 5–10 mg/kg, several are administered at 15 mg/kg and some as high as 30 mg/kg. Whether these antibodies might modulate other cellular processes, apart from angioinhibition, via FcγRI/c-Cbl signaling also merits future study. Such effects, if they occur, could be mitigated by the use of miniaturized configurations such as Fab or single chain variable fragments, fully deglycosylated antibodies, or Fc region engineering. Prolonged and frequent therapeutic IgG injections could potentially interfere with natural activation of FcγRs by endogenous IgGs. Therefore, targeted local therapy on an intermittent basis might be preferable for the treatment of chronic diseases.
Our bone marrow chimera experiments point to FcγRI on circulating myeloid cells as being critical for bevacizumab-induced angioinhibition. In human AMD as well as murine laser-induced angiogenesis, macrophages are highly spatially and temporally coincident with areas of choroidal neoangiogenesis.64,90 Indeed, of the various circulating myeloid cells in mice, only macrophages express FcγRI.91 Furthermore, we documented a reduction in macrophage infiltration following bevacizumab treatment that corresponds to angioinhibition. Nevertheless, in addition to disrupting Vegfr1 levels and signaling in macrophages, FcγRI-mediated events might also affect other myeloid cells, endothelial cells, or their bone marrow-derived precursors, and could transduce complex crosstalk among these cell types to modulate angiogenesis. Signaling pathways downstream of c-Cbl activation, as well as other yet to be determined molecular signals triggered via FcγRI, could be additionally responsible for human IgG1-induced angioinhibition.
Our data suggest that endogenous Igs could have a homeostatic role in modulating physiological or pathological angiogenesis. Future studies could explore the extent to which Igs regulate developmental vasculature. Polymorphisms in various FCGR genes have been associated with clinical responses to certain monoclonal antibodies in cancer.92 It would be interesting to explore whether variants in FCGR1 might affect the vascular status or clinical response of patients to various human IgG1 antibodies, when they are administered at doses that would be expected to induce FcγRI-mediated signaling.
Our studies, which have identified an unexpected vascular effect of widely used drugs, highlight the importance of employing rigorous biological controls for studies of IgGs. In revealing the intrinsic anti-angiogenic capacity of Fc-containing human IgG1s, these findings could be instructive in the future design and use of antibody-based therapeutics, expand understanding of the biological links between immunity and angiogenesis, and potentially enable novel angioinhibitory therapies.
Acknowledgments
We thank T.S. Khurana, S. Bondada, K. Ambati, A.M. Rao and G.S. Rao for discussions; L. Toll, G.R. Pattison R. King, L. Xu, M. McConnell, C. Payne, D. Robertson, G. Botzet, A. Uiettenbogaard and the IGB animal house and integrated microscopy facilities, for technical assistance. We thank J.V. Ravetch and N. Ferrara for generously sharing genetically modified mice. JA was supported by NIH grants DP1GM114862, R01EY018350, R01EY018836, R01EY020672, R01EY022238, R01EY024068, R21EY019778 and RC1EY020442, Doris Duke Distinguished Clinical Scientist Award, Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, Ellison Medical Foundation Senior Scholar in Aging Award, Foundation Fighting Blindness Individual Investigator Research Award, Carl Marshall Reeves Foundation, Harrington Discovery Institute Scholar-Innovator Award, John Templeton Foundation, Dr E. Vernon Smith and Eloise C. Smith Macular Degeneration Endowed Chair, and Research to Prevent Blindness departmental unrestricted grant; SDF by Associazione Italiana Ricerca sul Cancro (AIRC) grant no. IG11420 and Italian Ministry for Scientific Research, projects PON01_02342 and PON01_01434; MR and AS by Italian Ministry for Scientific Research, grants FIRB MERIT N° RBNE08NKH7_003 and PON01_01602, PON01_02342. JZB by NIH K08EY021521 and University of Kentucky Physician Scientist Award; BJF and SB by NIH T32HL091812 and UL1RR033173; YH by Alcon Research Award; AB-C by the Program for Advanced Medical Education (sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde and Fundação para a Ciência e Tecnologia, Portugal) and Bayer Global Ophthalmology Research Award; YH by Alcon Japan Research award; NK by Beckman Initiative for Macular Research and NIH K99/R00EY024336; TY by Fight for Sight Postdoctoral Award; CBW by International Retinal Research Foundation; BDG by American Heart Association and International Retinal Research Foundation; BKA by NIH R01EY017182 and R01EY017950, VA Merit Award, and Department of Defense.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Supplementary Information accompanies the paper on the Signal Transduction and Targeted Therapy website (http://www.nature.com/sigtrans)
JA is a co-founder of iVeena Holdings, iVeena Pharmaceuticals, iVeena Delivery Systems and Inflammasome Therapeutics, and has received honoraria from Allergan and research funding from Olix Pharmaceuticals unrelated to this work. JA and SDF are named as inventors on patent applications filed by the University of Kentucky relating to the technology described in this work. MRC is listed as an inventor on patents covering alemtuzumab and MRC and KLA are listed as inventors on patents covering the Fc mutated forms of alemtuzumab.
References
- Nelson AL , Dhimolea E , Reichert JM . Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9: 767–774. [DOI] [PubMed] [Google Scholar]
- Presta LG , Chen H , O'Connor SJ , Chisholm V , Meng YG , Krummen L et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997; 57: 4593–4599. [PubMed] [Google Scholar]
- Ferrara N , Hillan KJ , Gerber HP , Novotny W . Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391–400. [DOI] [PubMed] [Google Scholar]
- Group CR , Martin DF , Maguire MG , Ying GS , Grunwald JE , Fine SL et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WC , Wu X , Peale FV , Lee CV , Meng YG , Gutierrez J et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 2006; 281: 951–961. [DOI] [PubMed] [Google Scholar]
- Gerber HP , Wu X , Yu L , Wiesmann C , Liang XH , Lee CV et al. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc Natl Acad Sci USA 2007; 104: 3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L , Wu X , Cheng Z , Lee CV , LeCouter J , Campa C et al. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci 2008; 49: 522–527. [DOI] [PubMed] [Google Scholar]
- Manzano RP , Peyman GA , Khan P , Carvounis PE , Kivilcim M , Ren M et al. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin). Br J Ophthalmol 2007; 91: 804–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratviman-Storobinsky O , Lubin BC , Hasanreisoglu M , Goldenberg-Cohen N . Effect of subconjuctival and intraocular bevacizumab injection on angiogenic gene expression levels in a mouse model of corneal neovascularization. Mol Vis 2009; 15: 2326–2338. [PMC free article] [PubMed] [Google Scholar]
- Hashemian MN , Moghimi S , Kiumehr S , Riazi M , Amoli FA . Prevention and treatment of corneal neovascularization: comparison of different doses of subconjunctival bevacizumab with corticosteroid in experimental rats. Ophthalmic Res 2009; 42: 90–95. [DOI] [PubMed] [Google Scholar]
- Avisar I , Weinberger D , Kremer I . Effect of subconjunctival and intraocular bevacizumab injections on corneal neovascularization in a mouse model. Curr Eye Res 2010; 35: 108–115. [DOI] [PubMed] [Google Scholar]
- Dastjerdi MH , Saban DR , Okanobo A , Nallasamy N , Sadrai Z , Chauhan SK et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci 2010; 51: 2411–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkoyun I , Karabay G , Haberal N , Dagdeviren A , Yilmaz G , Oto S et al. Structural consequences after intravitreal bevacizumab injection without increasing apoptotic cell death in a retinopathy of prematurity mouse model. Acta Ophthalmol 2012; 90: 564–570. [DOI] [PubMed] [Google Scholar]
- Rabinowitz R , Priel A , Rosner M , Pri-Chen S , Spierer A . Avastin treatment reduces retinal neovascularization in a mouse model of retinopathy of prematurity. Curr Eye Res 2012; 37: 624–629. [DOI] [PubMed] [Google Scholar]
- Unkeless JC , Eisen HN . Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med 1975; 142: 1520–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV , Kinet JP . Fc receptors. Annu Rev Immunol 1991; 9: 457–492. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F , Ravetch JV . Divergent Immunoglobulin G subclass activity through selective Fc receptor binding. Science 2005; 310: 1510–1512. [DOI] [PubMed] [Google Scholar]
- Kim JH , Seo HW , Han HC , Lee JH , Choi SK , Lee D . The effect of bevacizumab versus ranibizumab in the treatment of corneal neovascularization: a preliminary study. Korean J Ophthalmol 2013; 27: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U , Harding SP , Rogers CA , Downes SM , Lotery AJ , Culliford LA et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013; 382: 1258–1267. [DOI] [PubMed] [Google Scholar]
- Carmeliet P . Angiogenesis in life, disease and medicine. Nature 2005; 438: 932–936. [DOI] [PubMed] [Google Scholar]
- Silver J . Drugs for macular degeneration, price discrimination, and Medicare's responsibility not to overpay. JAMA 2014; 312: 23–24. [DOI] [PubMed] [Google Scholar]
- Albuquerque RJ , Hayashi T , Cho WG , Kleinman ME , Dridi S , Takeda A et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med 2009; 15: 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WG , Albuquerque RJ , Kleinman ME , Tarallo V , Greco A , Nozaki M et al. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci USA 2009; 106: 7137–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler BJ , Gelfand BD , Kim Y , Kerur N , Tarallo V , Hirano Y et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 2014; 346: 1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T , Fowler BJ , Kim Y , Yasuma R , Krueger LA , Gelfand BD et al. Nucleoside reverse transcriptase inhibitors suppress laser-induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci 2015; 56: 7122–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y , Yasuma T , Mizutani T , Fowler BJ , Tarallo V , Yasuma R et al. IL-18 is not therapeutic for neovascular age-related macular degeneration. Nat Med 2014; 20: 1372–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couffinhal T , Silver M , Zheng LP , Kearney M , Witzenbichler B , Isner JM . Mouse model of angiogenesis. Am J Pathol 1998; 152: 1667–1679. [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME , Yamada K , Takeda A , Chandrasekaran V , Nozaki M , Baffi JZ et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 2008; 452: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y , Wiesmann C , Fuh G , Li B , Christinger HW , McKay P et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol 1999; 293: 865–881. [DOI] [PubMed] [Google Scholar]
- Marino M , Ruvo M , De Falco S , Fassina G . Prevention of systemic lupus erythematosus in MRL/lpr mice by administration of an immunoglobulin-binding peptide. Nat Biotechnol 2000; 18: 735–739. [DOI] [PubMed] [Google Scholar]
- Brown DM , Kaiser PK , Michels M , Soubrane G , Heier JS , Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ , Brown DM , Heier JS , Boyer DS , Kaiser PK , Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- Kami J , Muranaka K , Yanagi Y , Obata R , Tamaki Y , Shibuya M . Inhibition of choroidal neovascularization by blocking vascular endothelial growth factor receptor tyrosine kinase. Jpn J Ophthalmol 2008; 52: 91–98. [DOI] [PubMed] [Google Scholar]
- Takahashi H , Tamaki Y , Ishii N , Oikawa N , Mizuguchi E , Francis JH et al. Identification of a novel vascular endothelial growth factor receptor 2 inhibitor and its effect for choroidal neovascularization in vivo. Curr Eye Res 2008; 33: 1002–1010. [DOI] [PubMed] [Google Scholar]
- Takeda A , Baffi JZ , Kleinman ME , Cho WG , Nozaki M , Yamada K et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 2009; 460: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S , Pease ME , Wersinger DM , Masuda T , Vinores SA , Licht T et al. Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J Cell Physiol 2008; 217: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M , Sakurai E , Raisler BJ , Baffi JZ , Witta J , Ogura Y et al. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Invest 2006; 116: 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao MH , Morrison SL . Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol 1989; 143: 2595–2601. [PubMed] [Google Scholar]
- Walker MR , Lund J , Thompson KM , Jefferis R . Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J 1989; 259: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overdijk MB , Verploegen S , Ortiz Buijsse A , Vink T , Leusen JH , Bleeker WK et al. Crosstalk between human IgG isotypes and murine effector cells. J Immunol 2012; 189: 3430–3438. [DOI] [PubMed] [Google Scholar]
- Mancardi DA , Iannascoli B , Hoos S , England P , Daeron M , Bruhns P . FcγRIV is a mouse IgE receptor that resembles macrophage FcεRI in humans and promotes IgE-induced lung inflammation. J Clin Invest 2008; 118: 3738–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS , Nguyen C , Mendoza JL , Escandon E , Fei D , Meng YG et al. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther 1999; 288: 371–378. [PubMed] [Google Scholar]
- Bruhns P . Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119: 5640–5649. [DOI] [PubMed] [Google Scholar]
- Smith P , DiLillo DJ , Bournazos S , Li F , Ravetch JV . Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc Natl Acad Sci USA 2012; 109: 6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns P , Iannascoli B , England P , Mancardi DA , Fernandez N , Jorieux S et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113: 3716–3725. [DOI] [PubMed] [Google Scholar]
- Ben Mkaddem S , Hayem G , Jonsson F , Rossato E , Boedec E , Boussetta T et al. Shifting FcγRIIA-ITAM from activation to inhibitory configuration ameliorates arthritis. J Clin Invest 2014; 124: 3945–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloulou M , Ben Mkaddem S , Biarnes-Pelicot M , Boussetta T , Souchet H , Rossato E et al. IgG1 and IVIg induce inhibitory ITAM signaling through FcγRIII controlling inflammatory responses. Blood 2012; 119: 3084–3096. [DOI] [PubMed] [Google Scholar]
- van der Poel CE , Karssemeijer RA , Boross P , van der Linden JA , Blokland M , van de Winkel JG et al. Cytokine-induced immune complex binding to the high-affinity IgG receptor, FcγRI, in the presence of monomeric IgG. Blood 2010; 116: 5327–5333. [DOI] [PubMed] [Google Scholar]
- van der Poel CE , Spaapen RM , van de Winkel JG , Leusen JH . Functional characteristics of the high affinity IgG receptor, FcγRI. J Immunol 2011; 186: 2699–2704. [DOI] [PubMed] [Google Scholar]
- Barnes N , Gavin AL , Tan PS , Mottram P , Koentgen F , Hogarth PM . FcγRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity 2002; 16: 379–389. [DOI] [PubMed] [Google Scholar]
- Ioan-Facsinay A , de Kimpe SJ , Hellwig SM , van Lent PL , Hofhuis FM , van Ojik HH et al. FcγRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity 2002; 16: 391–402. [DOI] [PubMed] [Google Scholar]
- Bevaart L , Jansen MJ , van Vugt MJ , Verbeek JS , van de Winkel JG , Leusen JH . The high-affinity IgG receptor, FcγRI, plays a central role in antibody therapy of experimental melanoma. Cancer Res 2006; 66: 1261–1264. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y , Xiu Y , Komura K , Nimmerjahn F , Tedder TF . Antibody isotype-specific engagement of Fcγ receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med 2006; 203: 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh RS , Shi J , Jennings RM , Chappel JC , de Koning-Ward TF , Smith T et al. The importance of human FcγRI in mediating protection to malaria. PLoS Pathog 2007; 3: e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudino L , Nimmerjahn F , Azeredo da Silveira S , Martinez-Soria E , Saito T , Carroll M et al. Differential contribution of three activating IgG Fc receptors (FcγRI, FcγRIII, and FcγRIV) to IgG2a- and IgG2b-induced autoimmune hemolytic anemia in mice. J Immunol 2008; 180: 1948–1953. [DOI] [PubMed] [Google Scholar]
- Mancardi DA , Albanesi M , Jonsson F , Iannascoli B , Van Rooijen N , Kang X et al. The high-affinity human IgG receptor FcγRI (CD64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood 2013; 121: 1563–1573. [DOI] [PubMed] [Google Scholar]
- Guilliams M , Bruhns P , Saeys Y , Hammad H , Lambrecht BN . The function of Fcγ receptors in dendritic cells and macrophages. Nat Rev Immunol 2014; 14: 94–108. [DOI] [PubMed] [Google Scholar]
- Gul N , Babes L , Siegmund K , Korthouwer R , Bogels M , Braster R et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J Clin Invest 2014; 124: 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LM , Kim AC , Cattamanchi A , Ernst JD . Mycobacterium tuberculosis inhibits IFN-γ transcriptional responses without inhibiting activation of STAT1. J Immunol 1999; 163: 3898–3906. [PubMed] [Google Scholar]
- Bruhns P , Jonsson F . Mouse and human FcR effector functions. Immunol Rev 2015; 268: 25–51. [DOI] [PubMed] [Google Scholar]
- Jouvin-Marche E , Morgado MG , Leguern C , Voegtle D , Bonhomme F , Cazenave PA . The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics 1989; 29: 92–97. [DOI] [PubMed] [Google Scholar]
- Morgado MG , Cam P , Gris-Liebe C , Cazenave PA , Jouvin-Marche E . Further evidence that BALB/c and C57BL/6 γ2a genes originate from two distinct isotypes. EMBO J 1989; 8: 3245–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL , Shay T , Miller J , Greter M , Jakubzick C , Ivanov S et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012; 13: 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai E , Anand A , Ambati BK , van Rooijen N , Ambati J . Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2003; 44: 3578–3585. [DOI] [PubMed] [Google Scholar]
- Armour KL , van de Winkel JG , Williamson LM , Clark MR . Differential binding to human FcγRIIa and FcγRIIb receptors by human IgG wildtype and mutant antibodies. Mol Immunol 2003; 40: 585–593. [DOI] [PubMed] [Google Scholar]
- Idusogie EE , Presta LG , Gazzano-Santoro H , Totpal K , Wong PY , Ultsch M et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol 2000; 164: 4178–4184. [DOI] [PubMed] [Google Scholar]
- Shields RL , Namenuk AK , Hong K , Meng YG , Rae J , Briggs J et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem 2001; 276: 6591–6604. [DOI] [PubMed] [Google Scholar]
- Baudino L , Nimmerjahn F , Shinohara Y , Furukawa J , Petry F , Verbeek JS et al. Impact of a three amino acid deletion in the CH2 domain of murine IgG1 on Fc-associated effector functions. J Immunol 2008; 181: 4107–4112. [DOI] [PubMed] [Google Scholar]
- Baudino L , Shinohara Y , Nimmerjahn F , Furukawa J , Nakata M , Martinez-Soria E et al. Crucial role of aspartic acid at position 265 in the CH2 domain for murine IgG2a and IgG2b Fc-associated effector functions. J Immunol 2008; 181: 6664–6669. [DOI] [PubMed] [Google Scholar]
- Wynn TA , Chawla A , Pollard JW . Macrophage biology in development, homeostasis and disease. Nature 2013; 496: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV , Bolland S . IgG Fc receptors. Annu Rev Immunol 2001; 19: 275–290. [DOI] [PubMed] [Google Scholar]
- de Haij S , Jansen JH , Boross P , Beurskens FJ , Bakema JE , Bos DL et al. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer Res 2010; 70: 3209–3217. [DOI] [PubMed] [Google Scholar]
- Kyono WT , de Jong R , Park RK , Liu Y , Heisterkamp N , Groffen J et al. Differential interaction of Crkl with Cbl or C3G, Hef-1, and γ subunit immunoreceptor tyrosine-based activation motif in signaling of myeloid high affinity Fc receptor for IgG (FcγRI). J Immunol 1998; 161: 5555–5563. [PubMed] [Google Scholar]
- Park RK , Kyono WT , Liu Y , Durden DL . CBL-GRB2 interaction in myeloid immunoreceptor tyrosine activation motif signaling. J Immunol 1998; 160: 5018–5027. [PubMed] [Google Scholar]
- Shen H , Clauss M , Ryan J , Schmidt AM , Tijburg P , Borden L et al. Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood 1993; 81: 2767–2773. [PubMed] [Google Scholar]
- Barleon B , Sozzani S , Zhou D , Weich HA , Mantovani A , Marme D . Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996; 87: 3336–3343. [PubMed] [Google Scholar]
- Clauss M , Weich H , Breier G , Knies U , Rockl W , Waltenberger J et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 1996; 271: 17629–17634. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S , Minowa O , Kuno J , Noda T , Shibuya M . Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 1998; 95: 9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S , Sawano A , Nojima Y , Shibuya M , Maru Y . The c-Cbl/CD2AP complex regulates VEGF-induced endocytosis and degradation of Flt-1 (VEGFR-1). FASEB J 2004; 18: 929–931. [DOI] [PubMed] [Google Scholar]
- Thien CB , Blystad FD , Zhan Y , Lew AM , Voigt V , Andoniou CE et al. Loss of c-Cbl RING finger function results in high-intensity TCR signaling and thymic deletion. EMBO J 2005; 24: 3807–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes RA , Towers TL , Presta LG , Ravetch JV . Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6: 443–446. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F , Ravetch JV . Translating basic mechanisms of IgG effector activity into next generation cancer therapies. Cancer Immun 2012; 12: 13. [PMC free article] [PubMed] [Google Scholar]
- De Palma M , Lewis CE . Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013; 23: 277–286. [DOI] [PubMed] [Google Scholar]
- Hazenbos WL , Gessner JE , Hofhuis FM , Kuipers H , Meyer D , Heijnen IA et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in FcγRIII (CD16) deficient mice. Immunity 1996; 5: 181–188. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F , Lux A , Albert H , Woigk M , Lehmann C , Dudziak D et al. FcγRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci USA 2010; 107: 19396–19401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Merck T , Lammerts van Bueren JJ , Berger S , Rossen K , van Berkel PH , Derer S et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol 2010; 184: 512–520. [DOI] [PubMed] [Google Scholar]
- Duncan AR , Winter G . The binding site for C1q on IgG. Nature 1988; 332: 738–740. [DOI] [PubMed] [Google Scholar]
- Yasuma R , Cicatiello V , Mizutani T . Intravenous immune globulin suppresses angiogenesis in mice and humans. Signal Transduct Target Ther 2016; 1: (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J , Ellsworth JL , Hamacher N , Oak SW , Sun PD . Crystal structure of Fcγ receptor I and its implication in high affinity γ-immunoglobulin binding. J Biol Chem 2011; 286: 40608–40613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE , Cingle KA , Yoon YD , Ketkar N , L'Hernault N , Brown S . Correlation of histologic 2-dimensional reconstruction and confocal scanning laser microscopic imaging of choroidal neovascularization in eyes with age-related maculopathy. Arch Ophthalmol 2000; 118: 625–629. [DOI] [PubMed] [Google Scholar]
- Tan PS , Gavin AL , Barnes N , Sears DW , Vremec D , Shortman K et al. Unique monoclonal antibodies define expression of FcγRI on macrophages and mast cell lines and demonstrate heterogeneity among subcutaneous and other dendritic cells. J Immunol 2003; 170: 2549–2556. [DOI] [PubMed] [Google Scholar]
- Mellor JD , Brown MP , Irving HR , Zalcberg JR , Dobrovic A . A critical review of the role of Fcγ receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 2013; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.