Fig 7. Leg1a interacts with FGFR and glycosylation at N70 is crucial for Leg1 to interact with FGFR3 and to protect liver development under stress conditions.
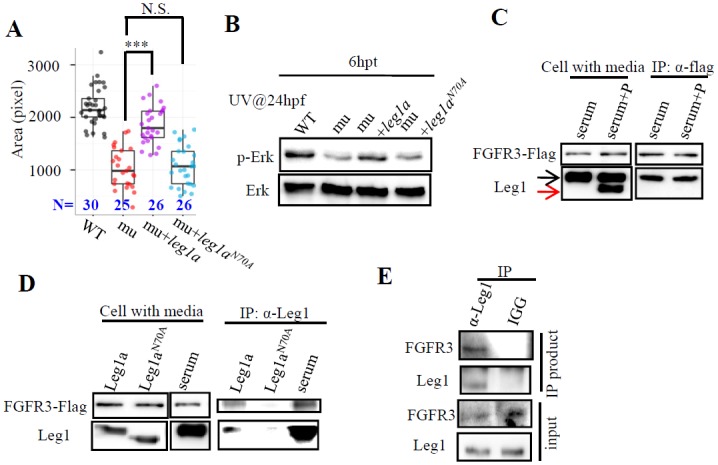
(A and B) Maternal-zygotic leg1azju1 embryos (mu) at one-cell stage were injected with leg1a or leg1bN70A mRNA. Injected embryos were treated with UV25 at 24 hpf and allowed to grow to 3.5 dpf for comparison of liver development by WISH using fabp10a probe (A) or to 30 hpf for protein extraction for western blot analysis of p-Erk and total Erk (B). A representative data set of three independent experiments was shown. ***, p<0.001, N.S., not significant. (C and D) Leg1 but not de-glycosylated Leg1 interacts with FGFR3. 293T cells over-expressing FLAG-tagged FGFR3 were incubated with total serum containing Leg1 (serum) or total serum Leg1 partially de-glycosylated by PNGase F (serum+P) (C), with supernatant containing secreted Leg1a or Leg1aN70A respectively from leg1a or leg1aN70A plasmids transfected 293T cells (D). In (C), black arrow, total Leg1, red arrow, de-glycosylated Leg1. An anti-FLAG antibody was used to perform the Co-IP, FGFR3 was detected with the anti-FLAG antibody and Leg1 with the Leg1 antibody. (E) Co-IP assay of interaction between Leg1a and FGFR3 in zebrafish. 200pg leg1 mRNA and 400 pgfgfr3 mRNA were co-injected into one-cell stage embryos. Total protein was harvested 7 hours after injection and was subjected to Co-IP using the Leg1 antibody or mouse IGG antibody. FGFR3 was detected by an anti-FRFR3 antibody. Western blot was repeated three times for (B), four times for (C), three times for (D) and (E).
