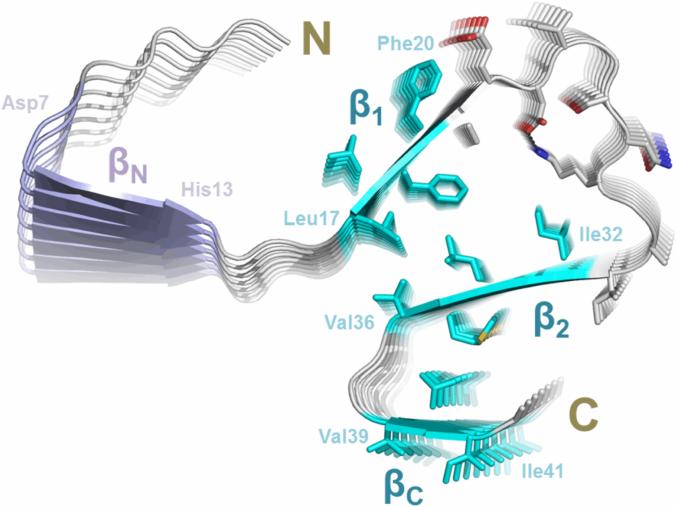
Figure 4. The structural model of Aβ42 fibrils.
A model of Aβ42 protofilament was built based on EPR restraints and Rosetta prediction. The side chains of residues 17-42 are shown in sticks, elucidating possible side chain interactions in the fibril core. The four stretches of strong exchange residues are colored based on how well they are structurally ordered. The three ordered regions, β1 (residues 17-20), β2 (residues 32-36) and βC (residues 39-41), are colored in cyan, while the less ordered region βN (residues 7-13) is in purple. The salt bridges between Asp23 and Lys28 are shown as black dashed lines.