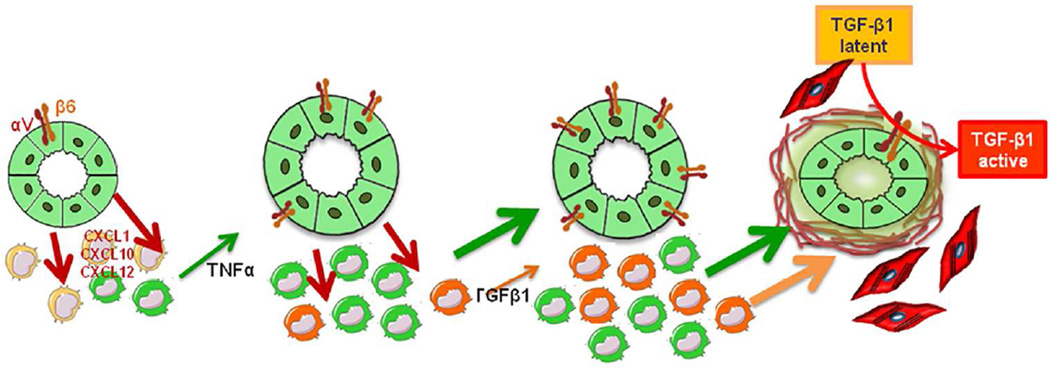
Figure 8. Working model: cross-talk between cholangiocytes and macrophages stimulates peribiliary fibrosis in Pkhd1del4/del4 mice.
In Pkhd1del4/del4 mice, portal fibrosis is the result of an intensive cross-talk between epithelial and inflammatory cells, originating from the FPC-deficient cholangiocytes. By secreting a range of chemokines, including CXCL1, CXCL10 and CXCL12, likely secondary to β-catenin signaling over-activation, Pkhd1del4/del4 cholangiocytes recruit macrophages in the portal area and stimulate their secretory functions. In the early phases, this portal infiltrate is mainly composed by M1 macrophages (green peribiliary cells), and TNFα (green arrow) is the predominant cytokine released until also TGFβ1 (orange arrow) becomes significantly secreted by M2 macrophages (orange peribiliary cells). Both macrophage-derived cytokines up-regulate αvβ6 integrin expression on biliary cysts and this, in turn, activates latent TGFβ1. Once activated, TGFβ1 induces production of collagen by cyst cholangiocytes, and as the disease progresses, by myofibroblasts, ultimately resulting in excessive matrix deposition into the peribiliary region.