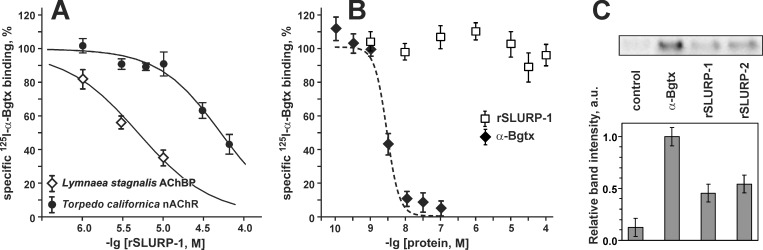
Fig 4. Competition of rSLURP-1 with 125I-α-Bgtx for binding to Ls-AChBP, membrane-bound nAChR from Torpedo californica, and α7-nAChR in the GH4C1 cell line.
(A). Each point is mean ± S.E. of three independent experiments. The Hill equation (Eq 1, with A1 = 0%) was fitted to normalized Ls-AChBP and T. californica data (% of control binding) for each of the three experiments independently. After averaging the following values for IC50 and nH were obtained 4.8 ± 0.9 μM and 0.92 ± 0.17 for Ls-AChBP, and 54 ± 15 μM and 1.3 ± 0.3 for T. californica nAChR (mean ± S.E., n = 3). Data for T. californica were taken from [23]. (B). Displacement of 125I-α-Bgtx by unlabeled Bgtx and rSLURP-1 from α7-nAChR in the GH4C1 cells. (C). Affinity purification of α7-nAChR subunit was performed using magnetic beads covalently coupled with rSLURP-1, rSLURP-2, and α-Bgtx or non-coupled beads (control) on GH4C1 cells overexpressing α7-nAChR (n = 2). The 40 μl samples of GH4C1 cells with final concentration of α-Bgtx-binding sites of 0.4 nM were used. The blots were analyzed by densitometry using ImageJ software (http://imagej.nih.gov/ij/).