Figure 1. Substitution of extracellular Na+ with NMDG+ increases TRPV1-mediated currents.
(A) Side view in ribbon representation of the transmembrane domains of two opposing TRPV1 subunits (as indicated by the black arrow, extracellular face on the top, intracellular face on the bottom) in the apo state (refined TRPV1 structural model [Bae et al., 2016]). The dashed boxes denote the location of the two constrictions proposed to serve as gates. Side chains of residues forming the binding site for capsaicin (purple) or determining activation of TRPV1 by protons (blue) are shown as sticks. (B) Representative time-course of whole-cell TRPV1-mediated currents elicited by 100-ms voltage pulses from -90 mV (triangles) to +90 mV (circles) at 300 ms intervals and at room temperature. The colored horizontal lines signal the onset of rapid-solution exchange as indicated by the labels. The dotted red line indicates the zero-current level. (C) Normalized TRPV1 current-voltage (I-V) relations obtained from 1s-duration voltage-ramps, following the same solution-exchange sequence as in (B). The darker curves are the mean and lighter-colored envelopes the standard error (n = 8).
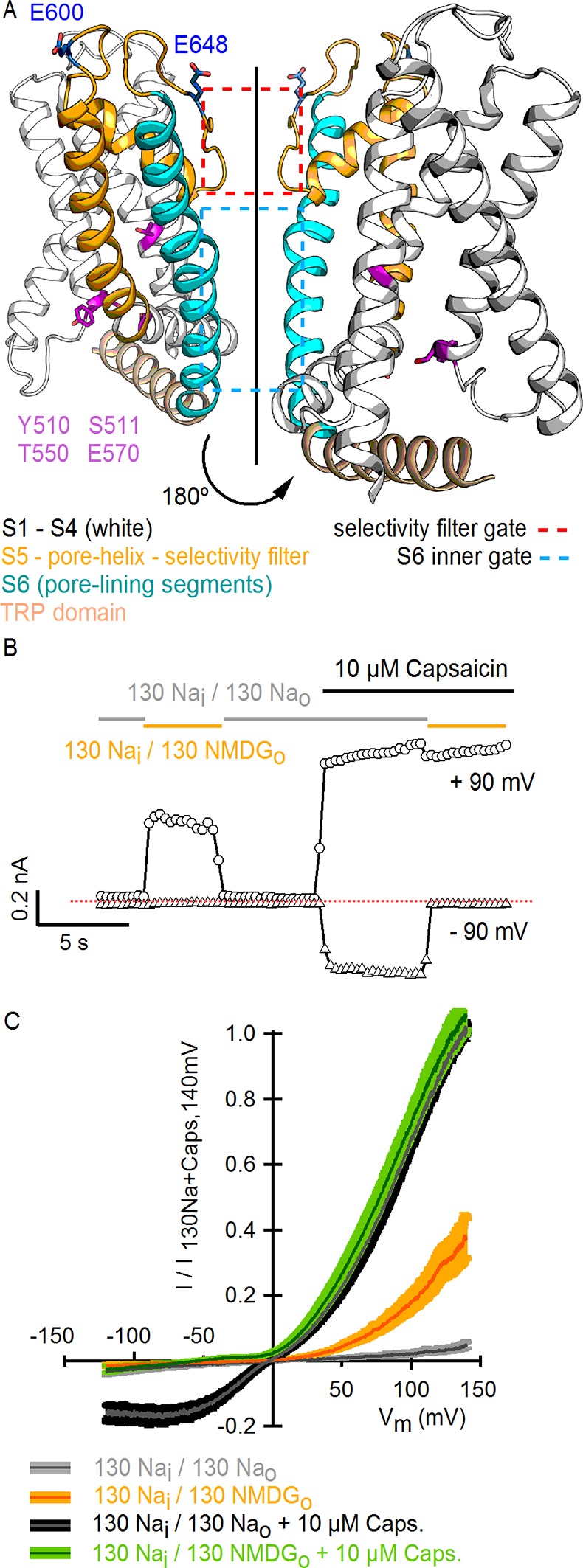
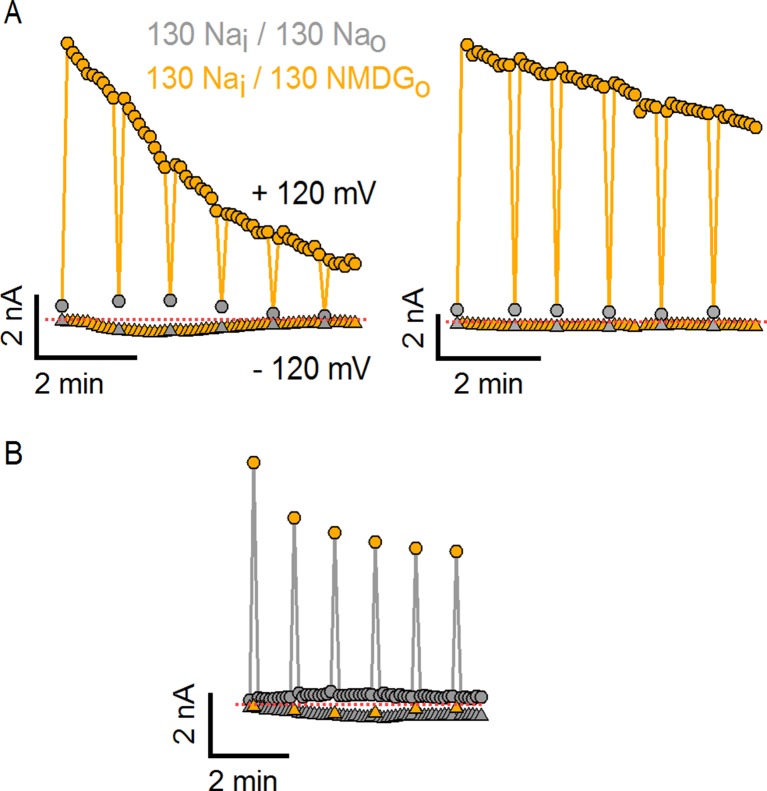
Figure 1—figure supplement 1. Substitution of external Na+ with NMDG+ induces channel rundown at room temperature with high cell-to-cell variability.
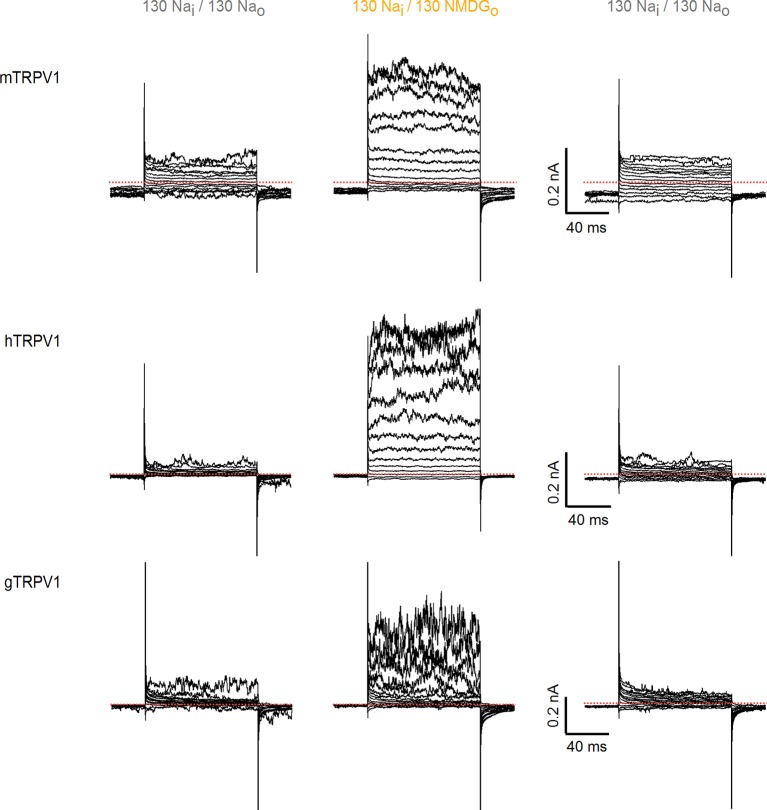
Figure 1—figure supplement 2. Activation of rat TRPV1 channel orthologues by substituting external Na+ with NMDG+.
Figure 1—figure supplement 3. Comparison of TRPV1 channel I-V relations measured using voltage steps and voltage ramps.
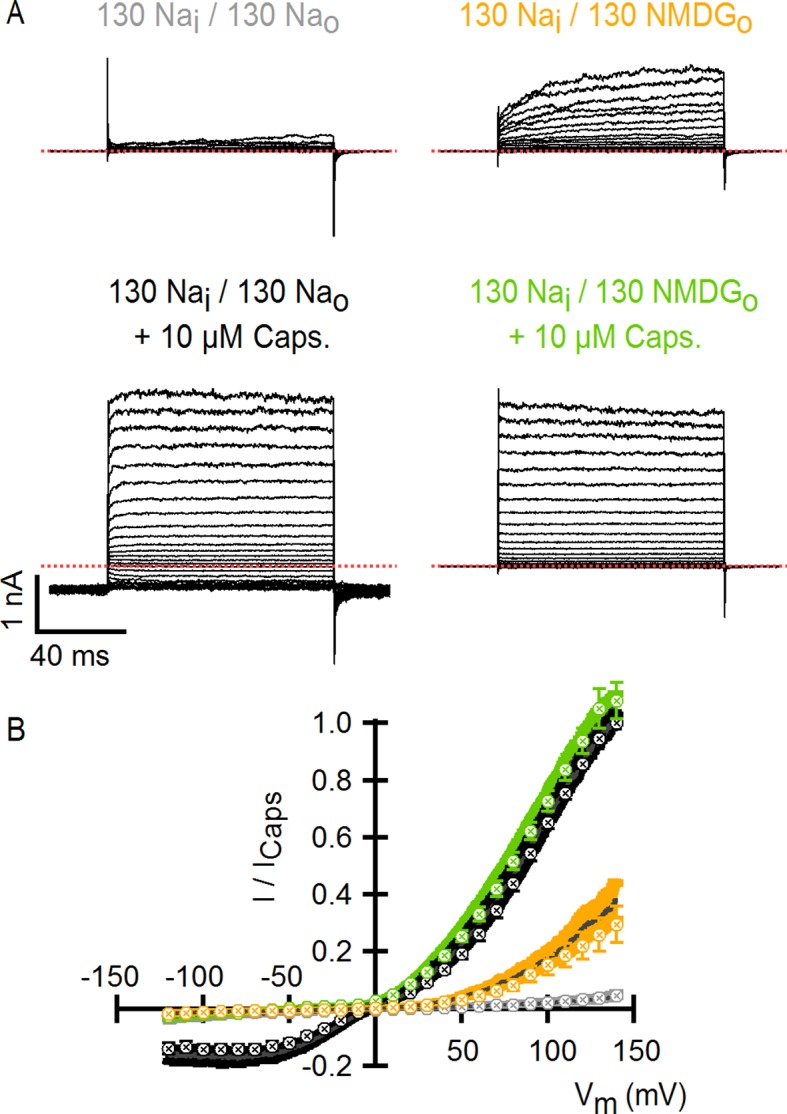
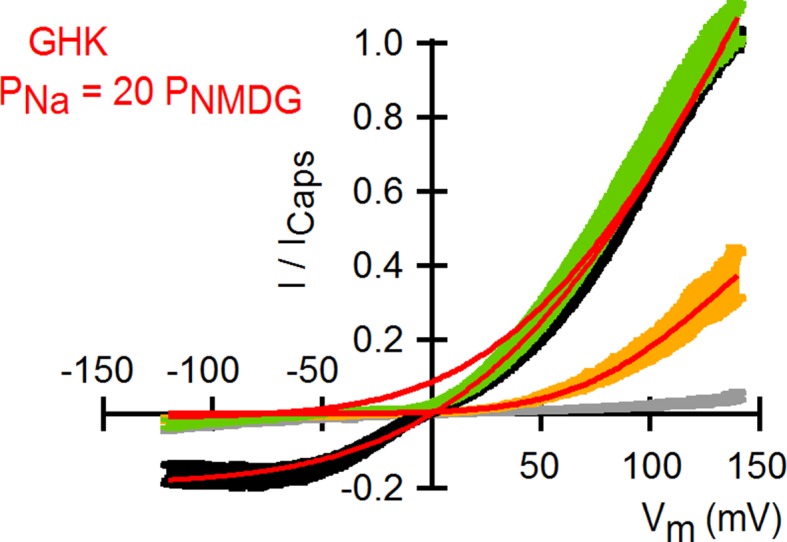
Figure 1—figure supplement 4. Theoretical I-V relations in the presence and absence of external Na+ obtained with the Goldman-Hodgkin-Katz current equation.