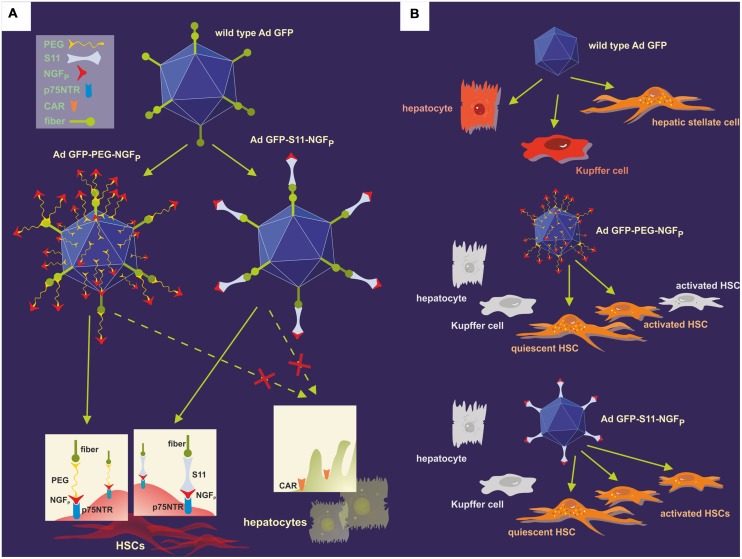
FIGURE 6.
Modification of the natural adenoviral tropism employing two different approaches resulted in the specific targeting of the p75NTR on HSCs. (A) The p75NTR-binding peptide NGFP and the wild-type Ad.GFP were covalently joined using PEGylation, thus forming Ad.GFP-PEG-NGFP (left) as well as merged with the help of S11 adapter molecules, resulting in Ad.GFP-S11-NGFP (right). The single chemical bonds of PEG and viral proteins are close together, thereby concealing both fiber knobs and RGD sequences on the viral surface, whereas the S11 fragment only binds to the fiber knobs, keeping the RGD sequences free for integrin interaction. Since fiber knobs are blocked in either case, binding to CAR, for example on hepatocytes, is inhibited and exclusive interaction of NGFP with p75NTR on the surface of HSCs can occur. (B) Wild-type Ad.GFP vectors can transduce hepatocytes, Kupffer cells, and HSCs in the liver, identifiable by the green fluorescence of GFP (top). As a result of the viral modifications, both Ad.GFP-PEG-NGFP (middle) and Ad.GFP-S11-NGFP (below) exclusively bound to and transfected quiescent and activated HSCs, but Ad.GFP-S11-NGFP showed an enhanced transduction efficiency concerning activated HSCs (below).