Abstract
The contribution of interleukin- (IL-) 4 receptor-alpha- (Rα-) dependent events in the pathogenesis of tuberculosis (TB) is controversial. We have recently shown IL-13 overexpression in mice to cause recrudescent Mtb replication and centrally necrotizing granulomas strongly resembling pathology of human TB. A deletion of IL-4Rα completely abrogates TB tissue pathology in these mice. To validate our results in human TB patients, we here determined the association of distinct variants of the IL4, IL13, IL4RA, IL13RA1, and IL13RA2 genes with cavity formation in a large Ghanaian cohort of HIV-negative individuals with newly diagnosed pulmonary TB. In fact, the structural variant of the IL4RA I50V, previously shown to result in enhanced signal transduction, was significantly associated with greater cavity size, and a variant of IL13RA2 was associated with disease in females. To evaluate whether the human-like TB pathology in IL-13-overexpressing mice is specifically mediated through the IL-4Rα subunit, we analyzed IL-13 transgenic mice with a genetic ablation of the IL-4Rα. In these mice, the IL-13-mediated increased susceptibility, human-like pathology of collagen deposition around centrally necrotizing granulomas, and alternative macrophage activation were abolished. Together, our genetic association study in human TB patients further supports the assumption that IL-13/IL-4Rα-dependent mechanisms are involved in mediating tissue pathology of human TB.
1. Introduction
Human tuberculosis (TB) is a leading global health threat and still constitutes a major medical challenge [1]. In 2014, the disease caused by Mycobacterium tuberculosis (Mtb) was responsible for 9.6 million new cases worldwide and 1.5 million deaths annually. TB is a systemic disease that becomes manifest most prominently in the lung [2]. Granuloma formation in mycobacterial infections characterizes the inflammatory tissue responses leading to containment of the pathogen. However, when a persistent Mtb reactivates within an initially protective granuloma, disease develops. In humans, this postprimary TB is associated with central granuloma necrosis followed by liquefaction of the caseous center, which erodes into the bronchus leaving a cavity and spreading Mtb into the environment. Consequently, granuloma necrosis and cavity formation are not only hallmarks of TB pathology, but also responsible for spreading infection. However, mechanisms leading to this typical tissue pathology during postprimary TB are not understood so far.
In human TB, an increased production of the T helper (TH)2 cytokines interleukin- (IL-) 4 and IL-13 is associated with lung damage [3–6], thus indicating that signals mediated through the common IL-4 receptor-alpha (Rα) may contribute to tissue pathology in postprimary TB. However, no functional connection between IL-4/IL-13 and the development of granuloma necrosis in TB patients has been made yet. In contrast, in experimental TB we have recently shown that overexpression of IL-13 in Mtb-infected mice results in recrudescent mycobacterial growth accompanied by centrally necrotizing granulomas strongly resembling pathology of human TB [7]. Therefore, IL-4Rα-mediated mechanisms appear to direct reactivation, granuloma necrosis, and cavity formation.
To further support our finding that the IL-4Rα is centrally involved in TB pathology, we here analyzed gene variants in the IL-4/-13-IL-4Rα pathway for their association with disease severity in a large Ghanaian TB cohort and continued validating our findings in experimental TB.
2. Methods
2.1. Study Participants, Genotyping, and Statistics
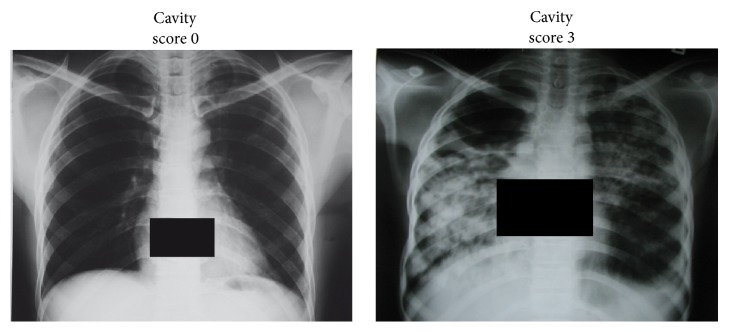
Patients with pulmonary TB and healthy control individuals were enrolled in Ghana, West Africa. Further details on the study group and the enrolment procedure of cases and controls are published [8] and described in Supplementary Material, Table S1, available online at http://dx.doi.org/10.1155/2016/4245028. All patients underwent posterior-anterior chest radiography. Pseudonymized films were read by two experienced radiologists. Cavities were individually assigned to the upper right, lower right, upper left, and lower left thoracic quadrants and overall assessed quantitatively being rated “0” (no lesion detectable), “1” (mild lesion), “2” (moderate lesion), and “3” (severe or multiple lesion) (Figure 1).
Figure 1.
Posterior-anterior chest X-ray of TB cases. Exemplary cavity score 0 (no cavities), cavity score 3 (extended, large cavities) scored blinded by an experienced radiologist, cross-checked by an experienced physician.
Genetic variants of the IL4, IL13, IL4R, IL13RA1, and IL13RA2 were selected due to evidence of association with and functional significance in the TH2-related condition asthma (IL4-589, rs2243250 [9]; IL13-1112, rs1800925 [10]; IL4R I50V, rs1805010 [11]; IL13RA1, rs2495636 [12]) and tissue pathology (IL13RA2, rs5946040 [13]). Further details on the genotyping protocol and statistical analysis are described in Supplementary Material. Multivariate logistic regression and ordinal logistic regression analyses were performed to determine influences of genetic variants on the disease status (cases versus controls) and the severity scores of radiological signs. Calculations included adjustments for age, gender, ethnicity, recruitment centers, and duration of cough, the latter because it was found correlated with the radiographic signs.
2.2. Mice
IL-13tg, IL-13tg × IL-4Rα −/− mice on a BALB/c genetic background were bred under specific-pathogen-free conditions at the Research Center Borstel. All experiments performed were in accordance with the German Animal Protection Law and were approved by the Animal Research Ethics Board of the Ministry of Environment, Kiel, Germany.
2.3. Bacteria and Infection of Experimental Mice
Mtb (H37Rv) was grown in Middlebrook 7H9 Broth (Difco, Detroit, MI) supplemented with Middlebrook OADC enrichment medium (Life Technologies, Gaithersburg, MI), 0.002% glycerol, and 0.05% Tween 80. Midlog phase cultures were harvested, aliquoted, and frozen at −80°C. After thawing, viable cell counts were determined by plating serial dilutions of the cultures on Middlebrook 7H10 agar plates followed by incubation at 37°C. Before infection of experimental animals, stock solutions of Mtb were diluted in sterile distilled water and pulmonary infection was performed using an inhalation exposure system (Glas-Col, Terre-Haute, IN). To infect mice with a low dose of 100 CFU/lung, animals were exposed for 40 min to an aerosol generated by nebulizing approximately 5.5 mL of a suspension containing 107 live bacteria. Inoculum size was checked 24 h after infection by determining the bacterial load in undiluted homogenates of the entire lung of infected mice.
2.4. Colony Enumeration Assay and Histology
Bacterial loads in lungs were evaluated at different time points after infection with Mtb to follow the course of infection. Organs from sacrificed animals were removed and prepared aseptically, weighed, and homogenized. Tenfold serial dilutions of organ homogenates were plated in duplicate onto Middlebrook 7H10 agar plates containing 10% OADC and incubated at 37°C for 19–21 days. One lung lobe per mouse was fixed in 4% formalin-PBS, set in paraffin blocks, and sectioned (2-3 μm). Histopathological analysis was performed using standard protocols for trichrome staining [14]. Immunohistochemical detection of arginase-1 was performed as previously described [15].
2.5. Quantitative Real-Time RT-PCR
Lung samples were isolated before and at different time points after aerosol infection with Mtb, weighed, and homogenized in 5 mL of 4 M guanidinium-isothiocyanate buffer. RNA extraction, reverse transcription, and real-time PCR were performed as previously published [16]. Primer and probe combinations selected from the Roche Universal Probe Library were used and are available upon request.
2.6. Determination of Arginase Activity
To determine arginase activity in murine tissue, weighed pieces of organs were homogenized in 100 μL of 0.1% Triton X-100 (Sigma) containing a protease inhibitor cocktail (Roche). 50 μL of 10 mM MnCl2 (Merck) and 50 mM Tris-HCl (Merck) were added to all samples and the enzyme was activated by heating for 10 min at 55°C. Arginine hydrolysis was conducted by incubating 25 μL of the activated lysate with 25 μL of 0.5 M L-arginine (Merck) at 37°C for 60 min. The reaction was stopped with 400 μL of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v). As a degree of arginase activity, the urea concentration was measured at 540 nm after addition of 25 μL α-isonitrosopropiophenone (Sigma; dissolved in 100% ethanol) followed by heating at 95°C for 45 min. One unit of arginase activity is defined as the amount of enzyme that catalyzes the formation of 1 μmol urea/min.
2.7. Statistical Analysis of Animal Experiments
Statistical analysis of data obtained in Mtb-infected mice was performed by nonparametric ANOVA with Dunn's Multiple Comparison Test.
2.8. Online Supplemental Material
The supplementary methods describe the enrolment of the study group. Table S1 illustrates the demographic and Table S2 the radiographic findings within the study group. Table S3 gives the genotype frequencies of variants of TB cases and controls.
3. Results and Discussion
3.1. In Human TB Patients, a Structural Variant of the Gene Encoding IL-4Rα Is Associated with Increased Cavity Size or Number
Based on cytokine determinations in TB patients, an IL-4Rα-mediated TH2 immune response has been implicated in driving TB tissue damage [3]. Particularly, IL-4 levels in peripheral blood mononuclear or bronchoalveolar lavage cells were found increased in TB patients [4, 17–25] and associated with granuloma necrosis and cavity formation [5]. To evaluate whether IL-4-/IL-13-mediated mechanisms are associated with pathology in human TB on a more functional level, we compared the influence of selected genetic variants of the IL4, IL13, IL4R, IL13RA1, and the IL13RA2 genes on the severity of pulmonary lesions in a large Ghanaian cohort of HIV-negative patients with pulmonary TB, comprising a total of 1971 cases and 2332 controls (Tables S1–S3). The frequencies of all genetic variants tested were in Hardy-Weinberg equilibrium among cases and controls. No association of the severity of radiographic findings (Figure 1) was observed with the variants of the IL4, IL13, IL13RA1, and IL13RA2 genes (Table 1). In striking contrast, the IL4R genotype A/A (I50V), when compared to the genotypes A/G and G/G, occurred more frequently among patients with high radiological severity scores for cavities compared to those with low ones (odds ratio (OR) 1.32, 95% confidence interval (CI) 1.0–1.7, p value 0.05). When assuming a recessive mode of inheritance, a similar OR with a higher significance (OR 1.33, CI 1.1–1.7, p value 0.016) was evident (Table 1, figures followed by asterisk). The finding indicates that a structural variant of the α chain of the IL-4/IL-13 receptor, which was shown to be associated with enhanced signal transduction [11], is associated with large or a greater number of cavities. Statistical calculations to address the question of whether the variants might be of any relevance in a case-control comparison revealed that the IL13RA2 genotype TT of the variant rs5946040 was, in females, significantly associated with occurrence of disease (OR 1.14, CI 1.1–2.1, p value 0.02; Table S3).
Table 1.
Genotype frequencies of variants of the IL4, IL4R, IL13, IL13RA1, and IL13RA2 genes in TB cases stratified for the size and number of cavities in chest radiographsA.
| Gene variant | Cavity score | OR | 95% CI | p | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||||
| IL4-589 rs2243250 | N | 79 | 632 | 509 | 186 | |||
| CC | 63.3% | 55.7% | 54.2% | 57.0% | 1 | |||
| CT | 32.9% | 37.7% | 38.9% | 37.1% | 0.96 | 0.6–1.5 | 0.85 | |
| TT | 3.8% | 6.6% | 6.9% | 5.9% | 0.89 | 0.6–1.3 | 0.58 | |
|
| ||||||||
| IL13-1112 rs1800925 | N | 81 | 649 | 517 | 189 | |||
| CC | 29.6% | 29.7% | 34.0% | 28.0% | 1 | |||
| CT | 51.9% | 50.4% | 49.9% | 53.5% | 1.00 | 0.8–1.3 | 0.97 | |
| TT | 18.5% | 19.9% | 16.1% | 18.5% | 0.86 | 0.6–1.1 | 0.31 | |
|
| ||||||||
| IL4R I50V rs1805010 | N | 82 | 649 | 518 | 191 | |||
| GG | 28.0% | 26.0% | 24.1% | 25.1% | 1 | |||
| AG | 56.1% | 51.5% | 51.5% | 45.0% | 0.99 | 0.8–1.3 | 0.92 | |
| AA | 15.9% | 22.5% | 24.3% | 29.8% | 1.32 | 1.0–1.7 | 0.05 | |
| GG/AG∗ | 84.2%∗ | 77.5%∗ | 75.7%∗ | 70.2%∗ | 1∗ | |||
| AA∗ | 15.9%∗ | 22.5%∗ | 24.3%∗ | 29.8%∗ | 1.33∗ | 1.1–1.7∗ | 0.016∗ | |
|
| ||||||||
| IL13RA1 rs2495636 | N | 27 | 226 | 140 | 51 | |||
| Female | ||||||||
| AA | 88.9% | 86.3% | 85.7% | 84.3% | 1 | |||
| AG | 11.1% | 13.7% | 14.3% | 11.8% | 1.17 | 0.7–2.0 | 0.55 | |
| GG | 0.0% | 0.0% | 0.0% | 3.9% | nc | |||
|
| ||||||||
| IL13RA2 rs5946040 | N | 27 | 227 | 140 | 51 | |||
| Female | ||||||||
| GG | 48.1% | 47.1% | 47.9% | 31.4% | 1 | |||
| GT | 44.4% | 40.1% | 43.6% | 52.9% | 1.26 | 0.9–1.9 | 0.24 | |
| TT | 7.4% | 12.8% | 8.6% | 15.7% | 0.90 | 0.5–1.7 | 0.74 | |
|
| ||||||||
| IL13RA1 rs2495636 | N | 57 | 424 | 380 | 137 | |||
| Male | ||||||||
| A | 91.2% | 93.4% | 94.2% | 90.5% | 1 | |||
| G | 8.8% | 6.6% | 5.8% | 9.5% | 1.13 | 0.7–1.8 | 0.62 | |
|
| ||||||||
| IL13RA2 rs5946040 | N | 57 | 420 | 375 | 138 | |||
| Male | ||||||||
| G | 59.6% | 71.0% | 69.1% | 71.7% | 1 | |||
| T | 40.4% | 29.1% | 30.9% | 28.3% | 0.93 | 0.7–1.2 | 0.59 | |
AOdds ratios (OR) and 95% confidence intervals (CI) were calculated by ordinal logistic regression. nc, not calculable. As the IL13RA1 and the IL13RA2 genes are located on the X-chromosome, association testing was performed separately for males and females. Genotype frequencies of IL4R I50V in TB cases stratified for the size and number of cavities assuming a recessive mode of inheritance are followed by asterisk.
Together, by using this genetic approach in human TB we showed for the first time that a single nucleotide polymorphism (SNP) of the IL4RA gene, which is known to enhance signal transduction via the IL-4Rα [11], was significantly associated with increased cavity size in human TB patients. This particular SNP was not associated with overall resistance or susceptibility to TB in the same Ghanaian cohort, but a SNP in the IL13RA2 gene was weakly associated with disease in females (Table S3, bold figure). Whereas IL-4 mediates its effects through either type I IL-4R (IL-4Rα/common-gamma chain) or type II IL-4R (IL-4Rα/IL-13Rα1), IL-13 has been suggested to execute its IL-4Rα-dependent effects through type II IL-4R. Nevertheless, IL-13-dependent responses can also develop in the absence of IL-4Rα-mediated signaling through the IL-13Rα2 chain [26], which was originally thought to operate exclusively as a decoy receptor for IL-13 [27, 28]. Our genetic association study in human TB patients supports our assumption that IL-13/IL-4Rα-mediated mechanisms contribute to reactivation and granuloma necrosis in TB. However, because we identified human IL4RA and IL13RA2 gene variants that are associated with greater risk of cavity development or progression, we could not exclude that in addition to IL-4Rα- and IL-13Rα2-mediated signals other mechanisms may also contribute to the pathogenesis of human postprimary TB.
3.2. Absence of the IL-4Rα Abrogates the Increased Susceptibility of Mtb-Infected IL-13tg Mice
Our human genetic association study revealed a role for both IL-4Rα and the IL-13Rα2 in the pathogenesis and progression of TB. Because IL-13-dependent responses are transduced by IL-4Rα-containing type II IL-4R but may also develop in the absence of IL-4Rα-mediated signaling through IL-13Rα2 [26], we next analyzed the specific role of IL-4Rα in the development of pathology in a murine model. In contrast to humans, after aerosol Mtb infection neither wild type nor IL-4Rα −/− mice develop granulomas with central necrosis after aerosol Mtb infection [7, 29]. Because wild type mice do not appreciably express the IL-4Rα ligand IL-13 during experimental TB, we have recently established a model in which IL-13 is overexpressed after infection with Mtb [7]. In fact, Mtb-infected IL-13tg mice are highly susceptible and develop human-like centrally necrotizing granulomas. To analyze the impact of the IL-4Rα on disease development after Mtb infection, we crossed IL-13tg animals with IL-4Rα −/− mice [7]. As we have recently published [7], the bacterial loads in the lungs of IL-13tg mice were significantly increased during the course of experimental TB (Figure 2). Deletion of IL-4Rα abrogates the increased susceptibility of Mtb-infected IL-13tg mice (Figure 2). Moreover, we demonstrate an obligatory role for IL-4Rα in mediating IL-13-dependent progression of experimental TB and we may exclude (also not formally shown) a role for IL-13Rα2.
Figure 2.
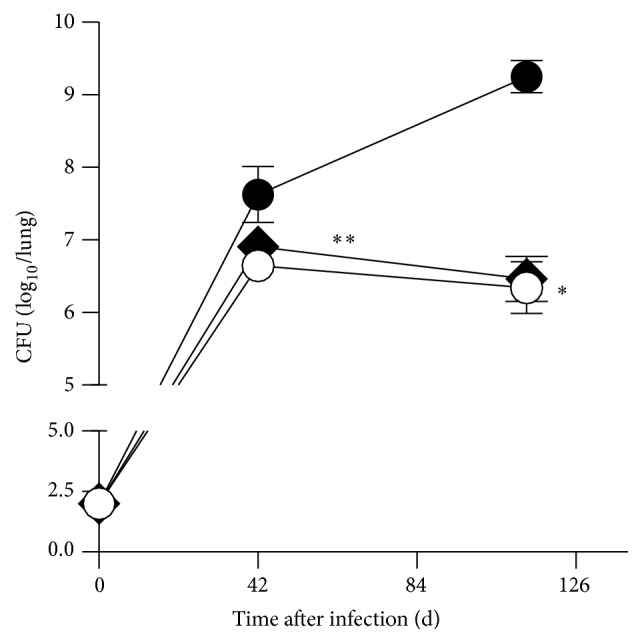
Decreased susceptibility of IL-13tg × IL-4Rα −/− mice to Mtb. BALB/c (white circles), IL-13tg (black circles), and IL-13tg × IL-4Rα −/− (black rhombs) mice were infected with 100 CFU Mtb H37Rv per aerosol. At different time points CFU was determined in lung homogenates. Data represent means and standard deviations of 4-5 mice (∗∗ p < 0.01 for comparison of IL-13tg and BALB/c mice; # p < 0.05 for comparison of IL-13tg and IL-13tg × IL-4Rα −/− mice; nonparametric ANOVA with Dunn's Multiple Comparison Test). One experiment representative of two performed is shown.
3.3. Collagen Deposition around Centrally Necrotizing Granulomas Is Abolished in IL-13tg Mice by Genetic Ablation of IL-4Rα
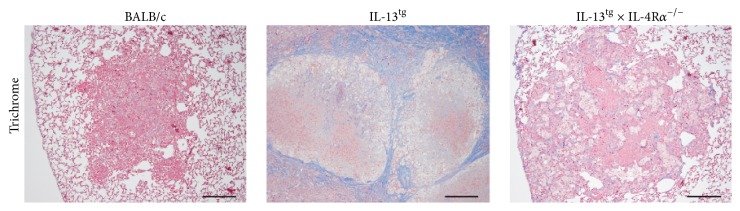
After aerosol infection with Mtb, circumscript mononuclear foci developed in wild type mice which progressively increased in size over time but never become necrotic [7]. In contrast, IL-13tg mice develop after infection with Mtb extensive pulmonary inflammation and centrally necrotizing granulomas and in these mice the deletion of IL-4Rα prevents granuloma necrosis as shown previously [7]. We also demonstrated that in Mtb-infected IL-13-overexpressing animals necrotic granulomas are surrounded by a collagen-rich fibrous layer, resembling human TB lesions [7]. To analyze the contribution of the IL-4Rα to this typical feature of TB pathology, we here infected BALB/c, IL-13tg, and IL-13tg × IL-4Rα −/− mice with Mtb via the aerosol route and evaluated after 113 days histopathological changes in lung sections stained for collagen (Figure 3). In lungs of Mtb-infected BALB/c mice, mononuclear cells accumulated but central granuloma necrosis could not be observed and collagen deposition in lung sections from wild type mice was hardly detectable. As recently published [7], IL-13tg mice developed massive granulomas with a necrotic core consisting of dead and dying cells demarcated by a fibrous capsule-like layer (Figure 3). The absence of the IL-4Rα completely stopped collagen deposition around centrally necrotizing granulomas (Figure 3). Together, we give further evidence that the IL-4Rα is involved in promoting key features of TB pathology in a murine model of central granuloma necrosis (and may thereby exclude a contribution of the IL-13Rα2).
Figure 3.
Collagen deposition around centrally necrotizing granulomas is abolished in IL-13tg mice by genetic ablation of the IL-4Rα. BALB/c, IL-13tg, and IL-13tg × IL-4Rα −/− mice were infected with 100 CFU Mtb H37Rv per aerosol and lung sections stained with trichrome were histologically evaluated after 113 days (n = 4-5). Whereas BALB/c mice did not develop necrotic lesions, central granuloma necrosis was found in lungs of IL-13tg mice surrounded by a fibrous rim. Deletion of the IL-4Rα abolished granuloma necrosis and collagen deposition in IL-13tg mice. Representative photomicrographs of one experiment out of two performed are shown.
As for our genetic association study in human subjects, we could not exclude that in addition to IL-13/IL-4Rα-mediated signals other mechanisms also contribute to the development of central granuloma necrosis in Mtb-infected mice. Initially, the question why Mtb-infected wild type mice do not develop central granuloma necrosis, the typical pathology in human postprimary TB was exciting. Whereas wild type mice do not express appreciable levels of IL-4 and IL-13 after infection with Mtb, an increased production of these TH2 cytokines is associated with lung damage in TB patients [3–6]. Consequently, Mtb infection of mice that overexpress IL-13 resulted in recrudescent mycobacterial growth accompanied by centrally necrotizing granulomas strongly resembling pathology of human TB [7]. Although our data indicate that the IL-13/IL-4Rα axis contributes to the development of central granuloma necrosis in TB, other mechanisms involved in the pathogenesis of TB may not be induced in the IL-13 transgenic mouse model. As for TH2 cytokines, these functions may be absent in Mtb-infected wild type mice but prominently expressed in human postprimary TB. However, overexpression of these functions still has these intrinsic limitations.
3.4. Obliterated Alternative Macrophage Activation in Mtb-Infected IL-13tg × IL-4Rα −/− Mice
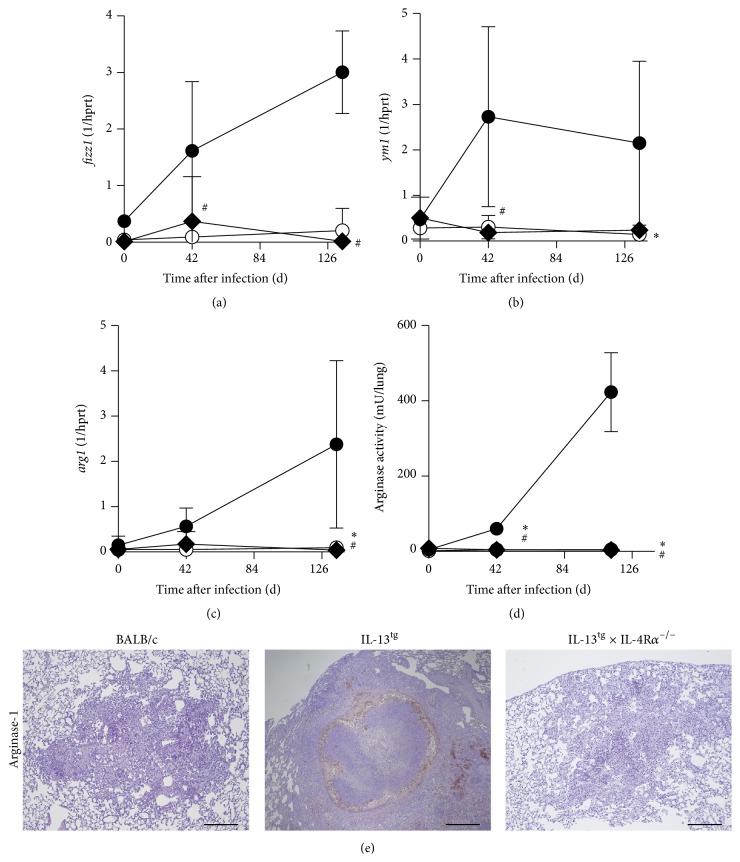
We have recently shown that in Mtb-infected IL-13tg mice Arg-1-expressing alternatively activated macrophages (aaMφ) are significantly induced and typically surround the necrotic centers of granulomas [7]. Specific elimination of Arg-1 in macrophages enhances antimycobacterial effector mechanisms in macrophages and leads to decreased lung bacterial loads during Mtb infection [15, 30]. Moreover, arginase activity contributes also to tissue remodeling and fibrosis in other diseases [31]. Because, in human TB, peripheral blood mononuclear cells from patients with pulmonary disease show higher arginase activity [32] and necrotic lesions are surrounded by fibrotic tissue [2], arginase activity in aaMφ may also contribute to TB pathology. To evaluate the effect of the IL-4Rα on the development of Arg-1 expressing aaMφ in IL-13tg mice, we infected BALB/c, IL-13tg, and IL-13tg × IL-4Rα −/− mice with Mtb via the aerosol route and analyzed alternative macrophage activation during the course of experimental TB (Figure 4). Gene expressions of fizz1 (Figure 4(a)) and ym1 (Figure 4(b)), two prototype markers for aaMφ, were hardly detectable in lung homogenates of wild type mice. As recently shown by us [7], fizz1 and ym1 were induced in IL-13tg mice to very high levels during the whole course of infection with Mtb (Figures 4(a) and 4(b)). The expression of these two markers for alternative macrophage activation was abolished in Mtb-infected IL-13tg × IL-4Rα −/− mice. In line with the virtually absent induction of fizz1 and ym1, arg1 mRNA was hardly detectable in wild type mice. But, in contrast, arg1 expression was significantly elevated in lungs from infected IL-13tg mice during the course of Mtb infection (Figure 4(c)). Importantly, in the absence of the IL-4Rα, arg1 gene expression was completely abrogated in lung homogenates of Mtb-infected IL-13tg mice (Figure 4(c)). In accordance with these findings, only low arginase activity was detectable in lung homogenates from wild type mice during the course of Mtb infection (Figure 4(d)). Whereas in IL-13tg mice arginase activity in lung homogenates increased during the course of infection, the deletion of the IL-4Rα abolished enzyme activity in these mice (Figure 4(d)). Immunohistochemical staining of lung sections for Arg-1 revealed that Arg-1 expression was undetectable in Mtb-infected wild type mice but was very prominent in lung granulomas of IL-13tg mice and necrotic centers were typically surrounded by Arg-1-expressing cells (Figure 4(e)) [7]. The deletion of IL-4Rα obliterated the expression of Arg-1 within the granulomas of Mtb-infected IL-13tg mice (Figure 4(e)). Together, these data show that after infection with Mtb alternative macrophage activation and Arg-1 expression in IL-13tg mice depend on the presence of the IL-4Rα and that IL-4Rα-mediated downstream mechanisms are putatively involved in TB pathology. This contention was recently supported by a report showing that, in patients with pulmonary TB, lung pathology was associated with increased arginase activity, which was further increased in TB patients coinfected with TH2 cytokine-inducing helminths [33]. Hence, in Mtb-infected human subjects, IL-13/IL-4Rα-induced Arg-1 may undermine macrophage effector responses, mediates collagen deposition and tissue remodeling, and thus contributes to tissue damage in human TB. Further genetic association studies and functional analyses should unravel the putative involvement of Arg-1 in the pathogenesis of human postprimary TB.
Figure 4.
In the absence of the IL-4Rα alternative macrophage activation is obliterated in Mtb-infected IL13tg mice. BALB/c (white circles), IL-13tg (black circles), and IL-13tg × IL-4Rα −/− (black rhombs) mice were infected with 100 CFU Mtb H37Rv per aerosol. At different time points, gene expression of (a) fizz1, (b) ym1, (c) arg1, and (d) arginase activity in lung homogenates of infected mice was determined. Results represent means and standard deviations of 4-5 mice (∗ p < 0.05 for comparison of IL-13tg and BALB/c mice; # p < 0.05 for comparison of IL-13tg and IL-13tg × IL-4Rα −/− mice; nonparametric ANOVA with Dunn's Multiple Comparison Test). One experiment representative of two performed is shown. (e) For immunohistological detection of Arg-1, lung sections were prepared from mice at 113 days after infection (n = 4-5). Arg-1 expression was not found in the lungs of Mtb-infected BALB/c mice but was prominent in granulomas of IL-13tg mice surrounding the necrotic center. Deletion of the IL-4Rα abolished Arg-1 expression in lungs of IL-13tg mice. Representative photomicrographs of one experiment out of two performed are shown.
4. Conclusions
These data support our assumption that IL-4Rα contributes to the development of central granuloma necrosis in human TB. However, IL-4Rα-dependent downstream mechanisms remain elusive and more functional analyses are required in the human system. Our animal study in IL-13tg × IL-4Rα −/− mice may point at aaMφ, which undermine antimycobacterial effector functions in macrophages and promote tissue remodeling and subsequently TB pathology. Further analysis in human TB and in the IL-13tg mouse model may unravel IL-4Rα-mediated functions involved in the pathogenesis of postprimary TB. More importantly, targeting the IL-4Rα or downstream mechanisms may constitute an approach to mitigate the development of postprimary TB. Because, for example, inhaling soluble IL-4R (altrakincept) was effective in the treatment of moderate asthma in patients [34], this biological may represent a candidate to medicate TB tissue pathology.
Supplementary Material
The supplementary methods describe the enrolment of the study group. Table S1 illustrates the demographic and Table S2 the radiographic findings within the study group. Table S3 gives the genotype frequencies of variants of TB cases and controls.
Acknowledgments
The authors thank Alexandra Hölscher, Johanna Volz, Kerstin Traxel, and Gabriele Röver for excellent technical assistance; Ilka Monath, Sven Mohr, and Claus Möller for organising the animal facility and taking care of the mice at the Research Center Borstel. They are also grateful to Heinz Fehrenbach for critically reading the paper. They are grateful to Frank Brombacher and Andrew McKenzie for providing IL-4Rα −/− and IL-13tg mice, respectively. The authors are indebted to the patients and control individuals who participated in the study. They thank all field workers, nurses, and physicians involved in the recruitment of patients and controls and gratefully acknowledge the excellent technical assistance of Lincoln Gankpala and Emmanuel Abbeyquaye and appreciate the logistics provided by the staff of the Kumasi Centre for Collaborative Research in Tropical Medicine, Kumasi, Ghana. This work was supported by a BMBF Grant 01KI0784, BMBF Grants NGFN1/01GS0162, NGFN2/NIE-S17T20, and NGFN-PLUS/01GS0811, the European Union Framework Program 7 (201483; TB-EUROGEN), and the German Center for Infection Research.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.WHO. Global Tuberculosis Report—2015. Geneva, Switzerland: World Health Organization; 2015. (edited by: WHO). [Google Scholar]
- 2.Ehlers S., Hölscher C. DTH-associated pathology. In: Kaufmann S. H., editor. Microbiology and Microbial Infections. London, UK: Arnold Publsihing; 2005. pp. 705–720. [Google Scholar]
- 3.Rook G. A. W. Th2 cytokines in susceptibility to tuberculosis. Current Molecular Medicine. 2007;7(3):327–337. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 4.Seah G. T., Scott G. M., Rook G. A. W. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. Journal of Infectious Diseases. 2000;181(1):385–389. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 5.Mazzarella G., Bianco A., Perna F., et al. T lymphocyte phenotypic profile in lung segments affected by cavitary and non-cavitary tuberculosis. Clinical and Experimental Immunology. 2003;132(2):283–288. doi: 10.1046/j.1365-2249.2003.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Crevel R., Karyadi E., Preyers F., et al. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. Journal of Infectious Diseases. 2000;181(3):1194–1197. doi: 10.1086/315325. [DOI] [PubMed] [Google Scholar]
- 7.Heitmann L., Dar M. A., Schreiber T., et al. The IL-13/IL-4Rα axis is involved in tuberculosis-associated pathology. Journal of Pathology. 2014;234(3):338–350. doi: 10.1002/path.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thye T., Browne E. N., Chinbuah M. A., et al. No associations of human pulmonary tuberculosis with Sp110 variants. Journal of Medical Genetics. 2006;43(7, article e32) doi: 10.1136/jmg.2005.037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenwasser L. J., Klemm D. J., Dresback J. K., et al. Promoter polymorphisms in the chromosome 5 gene cluster in asthma and atopy. Clinical and Experimental Allergy. 1995;25(supplement 2):74–78. doi: 10.1111/j.1365-2222.1995.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 10.van der Pouw Kraan T. C. T. M., van Veen A., Boeije L. C. M., et al. An IL-13 promoter polymorphism associated with increased risk of allergic asthma. Genes and Immunity. 1999;1(1):61–65. doi: 10.1038/sj.gene.6363630. [DOI] [PubMed] [Google Scholar]
- 11.Mitsuyasu H., Izuhara K., Mao X. Q., et al. Ile50Val variant of IL4R α upregulates IgE synthesis and associates with atopic asthma. Nature Genetics. 1998;19(2):119–120. doi: 10.1038/472. [DOI] [PubMed] [Google Scholar]
- 12.Heinzmann A., Mao X.-Q., Akaiwa M., et al. Genetic variants of IL-13 signalling and human asthma and atopy. Human Molecular Genetics. 2000;9(4):549–559. doi: 10.1093/hmg/9.4.549. [DOI] [PubMed] [Google Scholar]
- 13.Granel B., Allanore Y., Chevillard C., et al. IL13RA2 gene polymorphisms are associated with systemic sclerosis. The Journal of Rheumatology. 2006;33(10):2015–2019. [PubMed] [Google Scholar]
- 14.Herbert D. R., Hölscher C., Mohrs M., et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20(5):623–635. doi: 10.1016/S1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber T., Ehlers S., Heitmann L., et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. The Journal of Immunology. 2009;183(2):1301–1312. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hölscher C., Reiling N., Schaible U. E., et al. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. European Journal of Immunology. 2008;38(3):680–694. doi: 10.1002/eji.200736458. [DOI] [PubMed] [Google Scholar]
- 17.Dheda K., Chang J. S., Breen R. A., et al. In vivo and in vitro studies of a novel cytokine, interleukin 4δ2, in pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine. 2005;172(4):501–508. doi: 10.1164/rccm.200502-278oc. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez-Martínez M. C., Linares M., Báez R., et al. Intracellular expression of interleukin-4 and interferon-γ by a Mycobacterium tuberculosis antigen-stimulated CD4+ CD57+ T-cell subpopulation with memory phenotype in tuberculosis patients. Immunology. 2004;111(1):100–106. doi: 10.1111/j.1365-2567.2004.01785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dlugovitzky D., Bottasso O., Dominino J. C., et al. Clinical and serological studies of tuberculosis patients in Argentina receiving immunotherapy with Mycobacterium vaccae (SRL 172) Respiratory Medicine. 1999;93(8):557–562. doi: 10.1016/s0954-6111(99)90155-5. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez F. O., Rodriguez J. I., Agudelo G., Garcia L. F. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infection and Immunity. 1994;62(12):5673–5678. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith S. M., Klein M. R., Malin A. S., Sillah J., McAdam K. P. W. J., Dockrell H. M. Decreased IFN-γ and increased IL-4 production by human CD8+ T cells in response to Mycobacterium tuberculosis in tuberculosis patients. Tuberculosis. 2002;82(1):7–13. doi: 10.1054/tube.2001.0317. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S. K., Mitra D. K., Balamurugan A., Pandey R. M., Mehra N. K. Cytokine polarization in miliary and pleural tuberculosis. Journal of Clinical Immunology. 2002;22(6):345–352. doi: 10.1023/A:1020604331886. [DOI] [PubMed] [Google Scholar]
- 23.Marchant A., Amedei A., Azzurri A., et al. Polarization of PPD-specific T-cell response of patients with tuberculosis from Th0 to Th1 profile after successful antimycobacterial therapy or in vitro conditioning with interferon-α or interleukin-12. American Journal of Respiratory Cell and Molecular Biology. 2001;24(2):187–194. doi: 10.1165/ajrcmb.24.2.4274. [DOI] [PubMed] [Google Scholar]
- 24.Lienhardt C., Azzurri A., Amedei A., et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo . European Journal of Immunology. 2002;32(6):1605–1613. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Fenhalls G., Wong A., Bezuidenhout J., van Helden P., Bardin P., Lukey P. T. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infection and Immunity. 2000;68(5):2827–2836. doi: 10.1128/iai.68.5.2827-2836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fichtner-Feigl S., Strober W., Kawakami K., Puri R. K., Kitani A. IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nature Medicine. 2006;12(1):99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 27.Chiaramonte M. G., Donaldson D. D., Cheever A. W., Wynn T. A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. The Journal of Clinical Investigation. 1999;104(6):777–785. doi: 10.1172/jci7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood N., Whitters M. J., Jacobson B. A., et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor α 2. Journal of Experimental Medicine. 2003;197(6):703–709. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guler R., Parihar S. P., Savvi S., et al. IL-4Ralpha-dependent alternative activation of macrophages is not decisive for Mycobacterium tuberculosis pathology and bacterial burden in mice. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0121070.e0121070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Kasmi K. C., Qualls J. E., Pesce J. T., et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nature Immunology. 2008;9(12):1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesse M., Modolell M., La Flamme A. C., et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. The Journal of Immunology. 2001;167(11):6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 32.Zea A. H., Culotta K. S., Ali J., et al. Decreased expression of CD3ζ and nuclear transcription factor κB in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. Journal of Infectious Diseases. 2006;194(10):1385–1393. doi: 10.1086/508200. [DOI] [PubMed] [Google Scholar]
- 33.Monin L., Griffiths K. L., Lam W. Y., et al. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. Journal of Clinical Investigation. 2015;125(12):4699–4713. doi: 10.1172/jci77378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borish L. C., Nelson H. S., Lanz M. J., et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine. 1999;160(6):1816–1823. doi: 10.1164/ajrccm.160.6.9808146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary methods describe the enrolment of the study group. Table S1 illustrates the demographic and Table S2 the radiographic findings within the study group. Table S3 gives the genotype frequencies of variants of TB cases and controls.