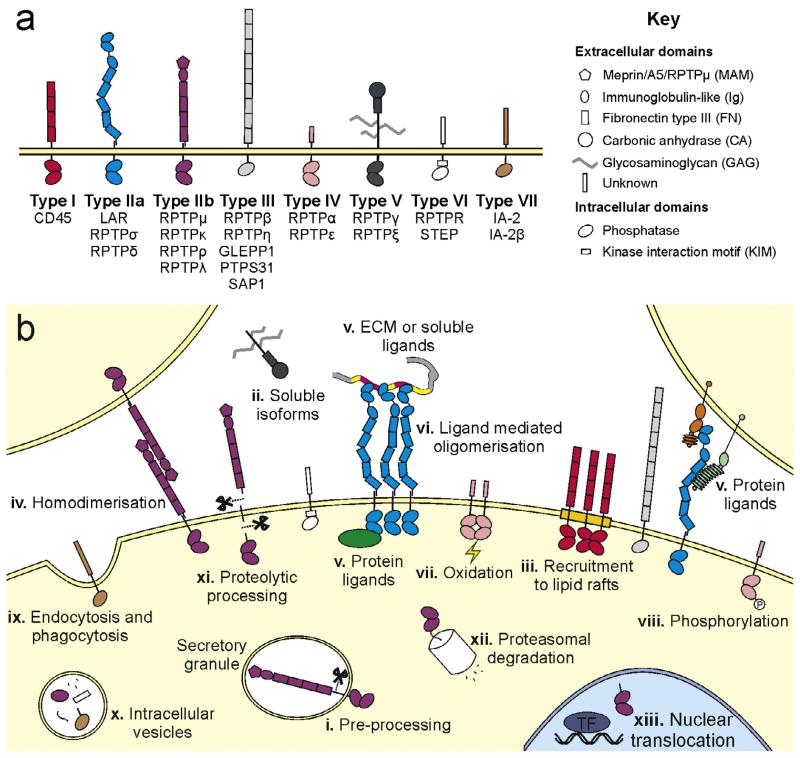
Fig. 1.
RPTP family classification, cellular localisation and regulation. (a) Classification of the human RPTPs, based on [1]. (b) Schematic depicting RPTP cellular location and modes of RPTP regulation: (i) the pro-proteins of both type IIa and IIb RPTPs are processed by subtilisin/kexin-like endoproteases in the trans-Golgi and subsequently presented at the cell surface as two non-covalently bonded subunits [35,36,131–134]. (ii) Alternative splicing occurs across the RPTP families, yielding multiple receptor isoforms [1]. The type V RPTP, RPTPζ, can also be expressed as a soluble isoform, more commonly known as phosphacan [135]. (iii) At the plasma membrane of activated T cells, the type I RPTP CD45, is recruited to lipid rafts [136]. (iv) Trans-homodimerisation of the type IIb RPTPs mediates the stability of adherens junctions [14,137]. (v) Although many of the RPTPs remain orphan receptors, the type IIa RPTPs have been shown to bind extracellular matrix (ECM) or soluble ligands [18,25,26] and cell surface proteins [27–29]. (vi) Ligand binding can potentially modulate clustering of RPTPs, for instance heparan sulphate proteoglycans (HSPGs) induce oligomerisation of type IIa RPTPs [58]. (vii) The generally inactive, membrane-distal phosphatase domain (D2) is thought to function as a redox sensor; oxidation of the D2 catalytic site cysteine regulates RPTPα (a type IV RPTP) dimerisation and phosphatase activity [138]. (viii) RPTPs may themselves be phosphorylated. For example Tyr and Ser phosphorylation mediates the activity of RPTPα and its interaction with intracellular signalling proteins [139,140]. (ix) RPTPs can be retrieved from the plasma membrane by endocytosis; the type VIII (pseudo-phosphatase) RPTP IA-2β contains a YXXφ motif within the cytoplasmic tail, targeting the receptor for clathrin-mediated endocytosis [141]. Alternatively, the type IIa RPTP, RPTPσ has been found to regulate autophagy and (x) to be located in autophagic vesicles targeted for lysosomal degradation [142]. (xi) Mature types IIa and IIb RPTPs at the cell surface can undergo proteolytic processing events to shed ectodomain fragments into the extracellular environment, followed by release of the intracellular tandem phosphatase domains [133,143]. (xii) This intracellular fragment may subsequently be targeted for proteosomal degradation. (xiii) However, it has been demonstrated that the intracellular fragment of RPTPκ and RPTPρ may also be imported into the nucleus and regulate transcription via dephosphorylation of transcriptional activators (TF) such as β-catenin and STAT3 [133,144].