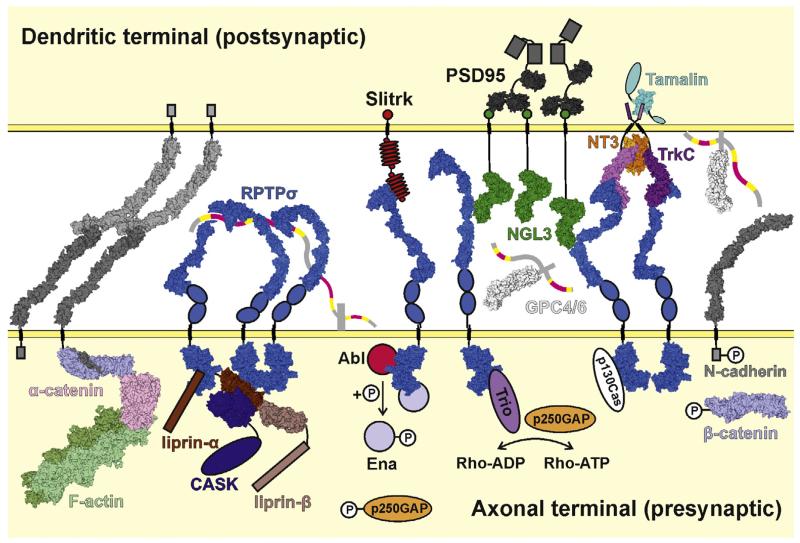
Fig. 5.
Model depicting synaptic interactions and signalling of type IIa RPTPs. The type IIa RPTP (RPTPσ illustrated) flexible ectodomains are able to adopt a variety of conformations [57]. For the RPTPσ N-terminal Ig domain to interact with presynaptic proteoglycans such as syndecan, more bent forms must be adopted (left), whereas to bind postsynaptic ligands such as TrkC, NGL-3, Slitrk and glypican, more extended conformations are required (right). HSPGs and soluble NT3 produce higher order RPTPσ arrays. RPTPσ can bind to presynaptic scaffolding proteins including liprin-α. Presynaptic RPTPσ signalling may also occur through dephosphorylation of N-cadherin and β-catenin and the stabilisation of adhesive N-cadherin:β-catenin:α-catenin:F-actin complexes or through several indicated cascades which culminate in activation of the Rho-GTPases and subsequent cytoskeletal remodelling. The same trans-synaptic interactions also lead to recruitment of postsynaptic density components such as PSD-95. Space filling models are derived from the following PDB accession codes and studies: human RPTPσ Ig1-2 (2YD3 [58]), Ig1-3 (2YD9 [58]), Ig1-FN3 (4PBX [57]; crystal structure used in full and divided into fragments to generate full range of ectodomain conformations; blue ovals represent FN4 and FN5 domains) and D1D2 (2FH7 [53]), mouse NGL-3 LRRIg1 (3ZYO [147]; green circles represent PDZ domain binding motifs), human RPTPσ Ig1-2:mouse TrkC LRRIg1 complex (4PBW [57]; aligned to 2IFG to generate model of RPTPσ:TrkC:NT3 complex), human TrkA LRR-Ig2:NGF complex (2IFG [148]; used to represent TrkC LRR-Ig2:NT3), rat tamalin PDZ domain (2EGO [149]; cyan ovals represent additional tamalin protein domains), human glypican-1 core protein (4AD7 [150]; heparan sulphate chains represented by ribbons and coloured as in Fig. 3b), mouse N-cadherin ectodomain (3Q2W [60]; grey boxes represent unbound N-cadherin intracellular tails, for clarity only one N-cadherin:α-catenin:β-catenin complex is shown), human α-catenin (4IGG [151]), zebrafish β-catenin (2Z6G [152]), mouse α-catenin:mouse β-catenin complex (4ONS [153], 4IGG and 2Z6G aligned to this crystal structure to obtain a more complete complex model), mouse E-caherin:β-catenin (1I7X [154], used to generate model of N-caderin intracellular domain with β-catenin), rabbit F-actin filament (3MFP [155]), rat PSD-95 PDZ-binding domains (3GSL [156] and 1TQ3), human liprin-α2 SAM1-3:mouse liprin-β1 SAM1-3 complex (3TAD [157]; brown boxes represent coiled-coil regions) and human CASK kinase domain:human liprin-α2 SAM1-3 complex (3TAC [157]; aligned with 3TAD to generate CASK:liprin-α:liprin-β model; blue oval represents remainder of CASK molecule). Structural information is currently lacking for Slitrk (red ovals, LRR repeats; small red circles, cysteine-rich caps; intracellular red circle, PDZ domain binding motif) and many HSPGs (grey stalk, protein core; heparan sulphate chains coloured as for glypican-1). Intracellular proteins signalling downstream of RPTPσ are represented as simple ovals or circles. Key phosphorylation sites are indicated by small white circles labelled ‘P’.