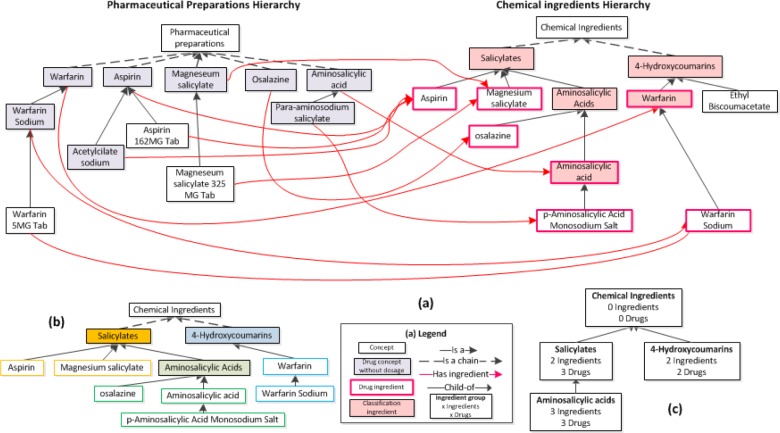
Figure 1.
(a) An excerpt of concepts from NDF-RT’s Pharmaceutical Preparations (PP) and Chemical Ingredients (CI) hierarchies. On the left, drug concepts in the PP hierarchy with no dosage information have a shaded background. On the right, seven drug ingredients are outlined in pink and five classification ingredients have a pink background. Two concepts, Aminosalicylic acid and Warfarin, are both drug ingredients and classification ingredients, i.e., they are dual use ingredients. Ethyl Biscoumacetate is neither a drug ingredient nor a classification ingredient. (b) CI revisited: Drug ingredients (not shaded) and their lowest common ancestor classification ingredients (shaded). Each drug ingredient is color-framed according to its lowest common ancestor classification ingredient. (c) The final IAbN for the excerpt of Figure 1(a). Ingredient groups are shown as white boxes that are labeled with the name of the lowest common ancestor from 1(b). Also shown are the total number of ingredient concepts summarized by the group, and the total number of drug concepts [with no dosage information in the PP hierarchy] with has ingredient roles pointing to the CI hierarchy. Child-of links between ingredient groups are shown as upward directed arrows.