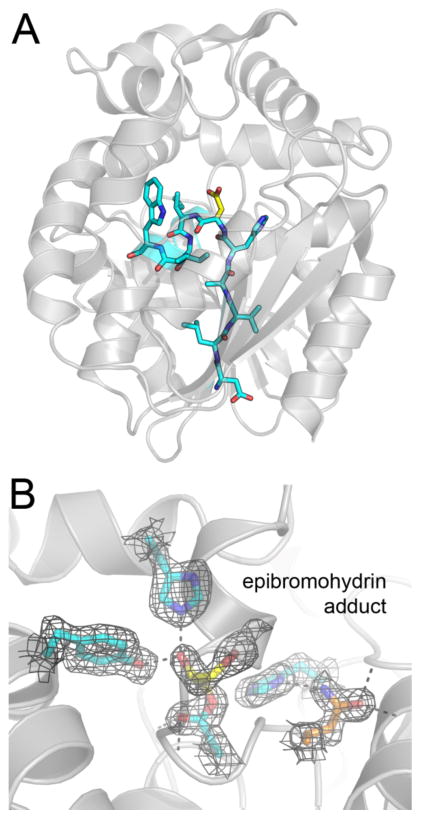
Figure 5. A covalent intermediate detected by mass spectrometry and X-ray crystallography.
(A) A Cif protomer is shown as a gray cartoon, with residues 124–133 shown as bold sticks with carbon atoms colored cyan. This peptide is produced by digesting Cif with chymotrypsin, and it spans the nucleophilic Asp129, highlighted by yellow colored carbon atoms. (B) Co-crystallization of Cif-E153Q with EBH results in an observable hydroxyalkyl-enzyme intermediate with ≥ 60% occupancy. The refined 2Fo-Fc map is shown as black mesh contoured to 1 σ. Side chains of catalytic active site residues and covalent adduct are shown with side chains as bold sticks; WT residues have carbon atoms colored cyan, mutant Gln153 is shown with carbon atoms colored orange, and the adduct has carbon atoms colored yellow.